Speak directly to the analyst to clarify any post sales queries you may have.
A strategic primer that frames cancer profiling as a convergent clinical and commercial priority driven by precision biomarkers and scalable laboratory transformation
Cancer profiling stands at a pivotal intersection of clinical demand, molecular innovation, and health system recalibration. Advances in sequencing, multiplexed proteomics, and refined sampling approaches have expanded the set of clinically actionable biomarkers, enabling clinicians and researchers to detect disease earlier, define heterogeneity with greater precision, and monitor therapeutic response in near real time. This introduction frames the contemporary landscape by connecting technological maturation with evolving stakeholder priorities across diagnostics laboratories, hospitals, pharmaceutical developers, and academic research centers.The clinical impetus for broad adoption of comprehensive profiling stems from increasing emphasis on personalized therapeutic strategies and more nuanced disease management pathways. Simultaneously, commercial adoption depends on operational scalability, sample accessibility, and clear reimbursement pathways that recognize the value of molecularly informed care. The introduction therefore situates profiling not merely as a laboratory capability but as a cross-functional enabler linking patient pathways, clinical trial design, and drug development strategies. By foregrounding these linkages, stakeholders can better prioritize investments in platforms, workflows, and partnerships that deliver measurable clinical and operational returns.
An analysis of rapid technological convergence and care delivery evolution reshaping cancer profiling practices and stakeholder value chains across clinical and commercial settings
The cancer profiling landscape is undergoing transformative shifts driven by convergence among technological innovation, clinical practice, and health-economics considerations. Long-read and short-read sequencing technologies are moving beyond research settings into clinical workflows, enabling more complete characterization of structural variants, complex rearrangements, and full-length transcript isoforms. Parallel advances in high-sensitivity assays such as digital PCR, refined immunohistochemistry panels, and multiplexed proteomic platforms are expanding the suite of analytes that inform diagnosis and therapy selection.These technological gains coincide with operational shifts: decentralized sampling models and noninvasive approaches like liquid biopsy are increasing patient access and enabling longitudinal monitoring outside the traditional clinic visit. At the same time, integration of bioinformatics pipelines and real-world data platforms is turning raw molecular outputs into clinically actionable reports, shortening the decision time for oncologists and clinical teams. Financial and regulatory systems are also adapting; payers are increasingly evaluating clinical utility over simple test volume, and regulatory frameworks are evolving to recognize adaptive diagnostics and companion tests. Collectively, these shifts are reshaping value chains, prompting incumbent laboratories and new entrants to redefine partnerships, and elevating the importance of robust clinical validation and interoperability as deciding factors for adoption.
A scenario-based assessment of how 2025 tariff shifts could propagate through procurement, investment decisions, and operational resilience in cancer profiling ecosystems
Anticipated tariff dynamics originating from major trade policy adjustments can generate cumulative impacts across the cancer profiling ecosystem, influencing costs, supply chains, and strategic sourcing decisions. When import duties or trade restrictions affect reagents, instrument components, or specialized consumables, laboratories and manufacturers can experience upward pressure on input costs. This, in turn, amplifies incentives to reassess supplier portfolios, expand local manufacturing capacity, or diversify procurement to jurisdictions with preferential trade terms. In practical terms, procurement teams will prioritize resilience and predictability, favoring suppliers that can demonstrate multiple supply routes and transparent pricing models.Beyond direct cost implications, tariffs can alter investment calculus for capital-intensive platforms. Clinical laboratories and hospital systems evaluating new sequencing or imaging platforms will weigh the stability of spare-part supply, lead times for maintenance, and total cost of ownership in light of potential trade-related price volatility. Pharmaceutical companies conducting biomarker-driven trials may re-evaluate site selection and central-lab strategies to mitigate the risk of disrupted assay turnaround times. To adapt, stakeholders are likely to accelerate supply chain mapping and scenario planning, negotiate longer-term supply agreements where possible, and explore reagent-agnostic or platform-agnostic assay designs that preserve clinical continuity should specific instruments or consumables become constrained. In short, tariff-driven friction favors flexibility, redundancy, and supplier transparency as critical operational priorities.
A comprehensive segmentation-driven perspective that maps clinical priorities, platform capabilities, and end-user operational requirements to enable differentiated commercialization strategies
Segmentation insights reveal distinct adoption pathways and value drivers when the market is viewed through the lenses of cancer type, technology, sample modalities, biomarker classes, application, and end user. Based on cancer type, profiling initiatives differentiate between hematologic malignancies and solid tumors; hematologic malignancies further focus on leukemia, lymphoma, and multiple myeloma where circulating tumor DNA and cell-free analyses often provide early diagnostic and monitoring advantages, whereas solid tumors emphasize tissue-based and liquid biopsy approaches across breast, colorectal, lung, and prostate cancer that require diverse assay sensitivities and tissue-context interpretation. These clinical nuances inform both assay selection and validation strategies.Based on technology, decision-makers compare fluorescence in situ hybridization, immunohistochemistry, microarray, next generation sequencing, and polymerase chain reaction, noting that next generation sequencing itself bifurcates into long read and short read approaches with distinct strengths for variant detection, structural resolution, and throughput. Based on sample type, blood, saliva, and urine introduce different pre-analytical workflows and sensitivity considerations, with blood subtypes such as plasma, serum, and whole blood each imposing specific extraction and stabilization requirements that influence assay performance. Based on biomarker type, DNA-based, protein-based, and RNA-based analyses complement each other to form orthogonal evidence for diagnosis, prognosis, and therapeutic selection. Based on application, diagnostic, monitoring, prognosis, research, and therapy selection use cases delineate end-to-end requirements; monitoring use cases often depend on liquid biopsy and minimal residual disease methods, while therapy selection relies on companion diagnostics and pharmacogenomic data to guide targeted therapies. Based on end user, diagnostic laboratories, hospitals, pharmaceutical companies, and research institutes prioritize different trade-offs between throughput, regulatory compliance, turnaround time, and integration with clinical workflows. The interplay among these segmentation axes guides product development, clinical validation roadmaps, and commercialization strategies, and stakeholders should design portfolios that align technological capability with the specific operational needs of target segments.
A regional intelligence framework highlighting how regulatory, reimbursement, and infrastructure differences shape adoption trajectories for cancer profiling across global markets
Regional insights underscore how geography shapes adoption patterns through regulatory regimes, reimbursement frameworks, and healthcare delivery architectures. In the Americas, established clinical lab networks and integrated healthcare systems drive early adoption of advanced molecular profiling, with centralized reference laboratories and academic centers often serving as innovation hubs that validate new assays and demonstrate clinical utility. These centers also shape payer engagement strategies and set precedents for reimbursement pathways that other institutions observe and emulate.Across Europe, Middle East & Africa, the landscape is heterogeneous: progressive regulatory agencies and well-resourced specialty centers accelerate adoption in certain markets, while others prioritize cost-effective, scalable diagnostics that fit decentralized or resource-limited settings. This diversity necessitates differentiated go-to-market approaches and adaptable pricing models. In the Asia-Pacific region, rapid investments in genomics infrastructure, rising domestic manufacturing capabilities, and strong public-private partnerships are expanding access to profiling technologies, while local clinical studies and population-specific biomarker research are generating regionally relevant evidence. Each region therefore requires tailored strategies that consider regulatory timelines, local clinical practice patterns, and the availability of specialized laboratory services.
A competitive dynamics narrative showing how integrated solutions, validation partnerships, and operational resilience determine leadership in cancer profiling markets
Competitive dynamics reflect an ecosystem of instrument manufacturers, assay developers, laboratory service providers, and software and data analytics firms. Leading players differentiate through integrated solutions that combine validated wet-lab workflows with robust bioinformatics and reporting capabilities, thereby reducing the time from sample collection to actionable clinical interpretation. Strategic partnerships between platform vendors and clinical laboratories accelerate clinical validation and create reference datasets that support payer engagement and guideline inclusion. New entrants often find traction by focusing on niche applications-such as minimal residual disease monitoring or platform-agnostic companion diagnostics-that address specific unmet clinical needs and bypass direct competition on broad, capital-intensive platforms.Intellectual property around assay chemistry, library preparation, and specialized reagents continues to influence competitive advantage, but access to clinical cohorts, turnaround guarantees, and quality management systems increasingly shape procurement decisions by hospitals and trial sponsors. Companies that can demonstrate interoperability, compliance with international laboratory standards, and clear clinical utility across multiple tumor types will maintain stronger negotiating positions. Additionally, firms that invest in scalable manufacturing and transparent supply chains reduce buyer risk and can capture preference in long-term procurement relationships. Overall, sustainable differentiation stems from coupling scientifically rigorous assays with resilient operations and clear pathways to clinical adoption.
A pragmatic set of strategic and operational recommendations enabling leaders to strengthen validation, supply resilience, reimbursement alignment, and clinical adoption pathways
Industry leaders should pursue a set of actionable moves that balance near-term operational resilience with long-term strategic positioning. Leaders need to prioritize assay validation across diverse sample types and clinical contexts to ensure that tests perform reliably in real-world settings; this includes cross-platform concordance studies and the development of robust pre-analytical protocols for plasma, serum, and whole blood as well as saliva and urine where appropriate. In parallel, investing in bioinformatics pipelines that provide transparent variant curation, clinical annotation, and report customization will help clinical teams interpret results quickly and reproducibly.Operationally, companies and laboratories should strengthen supply chain resilience by diversifying suppliers, pursuing local or regional manufacturing partners, and negotiating multi-year agreements that protect against episodic disruptions. Strategic partnerships with hospitals, academic centers, and pharmaceutical sponsors can accelerate clinical validation and create pathways for inclusion in clinical trials and guideline recommendations. From a go-to-market perspective, aligning reimbursement strategies with demonstrated clinical utility and engaging with payer stakeholders early can shorten adoption cycles. Finally, leaders should invest in workforce development and training to ensure that pathology, molecular laboratory personnel, and clinicians can integrate profiling outputs into routine care effectively, thereby maximizing clinical impact and commercial traction.
A robust mixed-methods research design combining stakeholder interviews, case studies, and evidence synthesis to produce validated insights for decision-makers
This research employs a multi-method approach that combines primary qualitative inquiry with rigorous secondary synthesis to produce actionable insights for stakeholders. Primary inputs include structured interviews with laboratory directors, clinical oncologists, procurement leaders, and regulatory advisors to capture decision criteria, validation practices, and operational constraints. These interviews are complemented by case studies of clinical implementations and collaborations between platform vendors and healthcare institutions to illustrate practical pathways from validation to routine use.Secondary analysis integrates peer-reviewed literature on assay performance and clinical utility, technical whitepapers on platform capabilities, and regulatory guidance documents that govern diagnostics and companion tests. Data triangulation ensures consistency across sources and highlights areas where evidence is robust versus where additional clinical validation is needed. Throughout the methodology, emphasis rests on transparency of assumptions, reproducibility of analytic steps, and the presentation of alternative scenarios to support strategic planning. Quality control measures include independent expert review and iterative validation of findings with external clinical and laboratory stakeholders.
A concluding synthesis emphasizing pathways to clinical integration, operational sustainability, and evidence-driven adoption of cancer profiling solutions
In conclusion, cancer profiling is transitioning from a predominantly investigational capability to a cornerstone of precision oncology workflows, driven by technological maturation, expanding biomarker portfolios, and evolving care models. The trajectory toward integrated diagnostics and longitudinal monitoring creates opportunities for stakeholders who can align technical performance with clinical validation, operational resilience, and payer engagement. Key barriers remain, including pre-analytical variability, interoperability challenges, and the need for broader real-world evidence to support reimbursement and guideline inclusion, but these challenges are addressable through targeted investment and collaborative validation efforts.Looking ahead, success will favor organizations that design modular platforms adaptable to varied clinical contexts, that invest in transparent data reporting and bioinformatics, and that cultivate strategic partnerships across laboratories, healthcare systems, and industry sponsors. The most impactful solutions will combine scientific rigor with pragmatic attention to supply chain stability, regulatory alignment, and clinician usability, enabling profiling to deliver measurable improvements in patient outcomes and more efficient clinical pathways.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Cancer Profiling Market
Companies Mentioned
The key companies profiled in this Cancer Profiling market report include:- 4basecare Onco Solutions Private Limited
- ACT Genomics Co., Ltd. by Prenetics Global Limited
- Agendia, Inc.
- Agilent Technologies, Inc.
- BostonGene Corporation
- Caris Life Sciences
- Exact Sciences Corporation
- F. Hoffmann-La Roche AG
- GENINUS Inc.
- Genomic Life
- GenScript Biotech Corporation
- Guardant Health, Inc.
- Hologic, Inc.
- HTG Molecular Diagnostics, Inc.
- Illumina, Inc.
- IMBdx, Inc.
- Laboratory Corporation of America Holdings
- Lucence Health, Inc.
- Merck KGaA
- NanoString Technologies, Inc.
- Neogenomics, Inc.
- Nonacus Limited
- OncoDNA S.A.
- Oncompass Medicine Hungary Kft.
- Paragon Genomics, Inc.
- Personalis, Inc.
- Perthera, Inc.
- Qiagen N.V.
- Renovaro Biosciences Inc.
- Strand Life Sciences
- Sysmex Corporation
- Takara Bio Inc.
- Tempus Labs Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
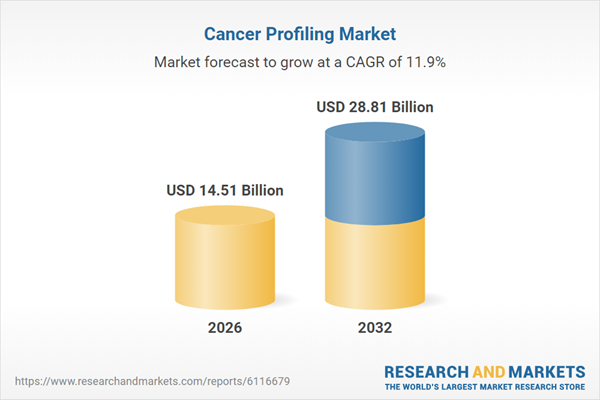
| Estimated Market Value ( USD | $ 14.51 Billion |
| Forecasted Market Value ( USD | $ 28.81 Billion |
| Compound Annual Growth Rate | 11.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 35 |