Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative framing of how precision detection technologies, regulatory demands, and operational priorities converge to redefine device selection, deployment, and lifecycle management
The field of ionizing radiation precision instruments spans a wide spectrum of devices and use cases, providing the essential measurements that underpin safety, clinical effectiveness, regulatory compliance, and industrial throughput. Advances in detection technologies and data integration have elevated these instruments from standalone meters to networked components of wider radiation management ecosystems. As organizations across healthcare, energy, defense, and industrial sectors confront evolving regulatory expectations and operational pressures, measurement accuracy, traceability, and ease of integration have become deciding factors in procurement and lifecycle planning.Manufacturers and end users alike must reconcile legacy platforms with emergent semiconductor-based detectors, modular telemetry systems, and improved calibration workflows. In parallel, attention to portability and fixed-installation roles shapes product development priorities, while certification cycles and cross-border supply chains influence time-to-deployment. Consequently, strategic choices around detector types, portability form factors, and embedded analytics now determine not only product differentiation but also the value delivered to clinical physicists, safety officers, and radiography teams.
This introduction frames the subsequent analysis by situating technology trends, regulatory drivers, and user needs within a single narrative. It establishes the imperative for firms to combine engineering excellence with commercial agility to support safer operations, more reproducible measurements, and operational continuity across diverse environments.
How advances in semiconductor detection, embedded analytics, supply chain diversification, and regulatory traceability are reshaping product design and commercial models across the instrumentation ecosystem
The landscape is shifting along several transformative axes that collectively alter competitive dynamics and adoption pathways. First, sensor innovation-particularly the maturation of semiconductor devices such as diamond and silicon detectors-has compressed the trade-off between sensitivity and form factor, enabling instruments that are simultaneously more compact, more durable, and easier to integrate into digital monitoring platforms. As these technologies scale, traditional ionization chambers and scintillation counters are being rethought for mixed-technology architectures where each detector class contributes specific strengths to a modular instrument suite.Second, the ubiquity of embedded processing and cloud-capable telemetry has elevated the role of software in delivering value. Signal processing algorithms, calibration firmware, and secure data pipelines now determine downstream usability more often than raw hardware specifications. Consequently, firms that pair detector hardware with robust analytics, traceable calibration workflows, and services models can command stronger operational relationships with hospitals, industrial sites, and research labs.
Third, geopolitical and supply chain pressures are prompting manufacturers to diversify sourcing and localize critical components, which in turn affects lead times and qualification cycles. Regulatory agencies are also tightening expectations around device traceability and environmental monitoring, pushing end users toward instruments that support audit-ready data exports and standardized calibration records. Taken together, these shifts favor agile engineering, partnerships across the semiconductor and instrumentation value chain, and a renewed emphasis on lifecycle services.
The enduring operational, procurement, and engineering consequences of cumulative tariff measures necessitate supply chain reengineering, component localization, and total-cost-of-ownership procurement approaches
Cumulative tariff actions implemented in the United States have introduced a complex overlay of cost, timing, and strategic response that manufacturers and purchasers must now navigate. Increased duties on certain imported components and finished instruments raise input costs for suppliers that rely on cross-border manufacturing nodes for detectors, readout electronics, and calibration equipment. These cost pressures tend to inflate procurement cycles, compel renegotiation of supplier contracts, and incentivize manufacturers to evaluate nearshoring, alternative vendors, or greater vertical integration to preserve margin and guarantee supply continuity.In parallel, procurement teams in healthcare and government continue to confront longer qualification timelines as alternate suppliers undergo validation and certification. Some vendors absorb tariff-related costs to maintain competitiveness, while others pass them downstream, prompting end users to reassess replacement cadences and maintenance budgets. The tariffs also accelerate efforts by manufacturers to redesign product architectures to reduce dependence on tariffed components, substitute materials where feasible, and prioritize interoperability so that validated detectors and readouts can be integrated with locally sourced subsystems.
From a strategic perspective, tariffs are catalyzing new partnerships between instrumentation firms and domestic electronics manufacturers, shifting R&D priorities toward scalable semiconductor processes that can be localized, and prompting procurement policies that explicitly account for total cost of ownership rather than unit price alone. These adaptations are reshaping supplier roadmaps and creating opportunities for firms that can demonstrate stable lead times, compliance-ready documentation, and clear plans for tariff mitigation.
A nuanced segmentation framework linking detector families, portability form factors, semiconductor subtypes, application-driven measurement needs, and distinct end-user procurement behaviors
A segmented view of the market clarifies where demand, product innovation, and service models intersect to create differentiated value. When analyzed by product type, instruments range from traditional Geiger-Muller counters and ionization chambers to scintillation detectors and advanced solid state detectors, each offering distinct performance profiles for sensitivity, energy discrimination, and environmental robustness. Considerations of portability create dichotomies between fixed installations that serve continuous monitoring and portable units designed for field surveys and rapid response, shaping design priorities such as ruggedization, battery management, and user interface simplicity.Technology segmentation further highlights the emergence of semiconductor devices alongside classical ionization chambers and scintillation counters; within semiconductor approaches, there is important differentiation between diamond detectors and silicon detectors, with each material offering trade-offs in radiation hardness, energy response, and operational lifetime. Application-driven segmentation reveals how measurement requirements differ across dosimetry, environmental monitoring, industrial radiography, nuclear safeguards, and radiation therapy, which in turn influences calibration standards, certification needs, and software functionality. Finally, end-user segmentation underscores divergent procurement behaviors: hospitals and nuclear stations prioritize traceability and long-term service contracts, industrial facilities value ruggedness and ease of calibration, and research institutes-split between academic labs and government labs-demand experimental flexibility and high-performance specifications.
Integrating these segmentation lenses allows manufacturers and purchasers to align product roadmaps with use-case-driven specifications, prioritize regulatory certification pathways, and tailor aftermarket services to the distinct lifecycle expectations of each customer cohort.
How divergent regulatory frameworks, procurement practices, and regional capacity-building priorities across the Americas, EMEA, and Asia-Pacific are shaping adoption pathways and vendor strategies
Regional dynamics imprint strong differences in regulatory environments, procurement norms, and technology adoption rates that influence where investment and innovation concentrate. In the Americas, end users emphasize operational continuity and regulatory compliance across clinical and nuclear sectors; there is a notable appetite for modular systems that support remote calibration and cloud-enabled incident response, as well as strong demand from research institutes pursuing high-precision detectors for experimental programs.Across Europe, the Middle East, and Africa, regulatory harmonization efforts and stringent environmental monitoring priorities shape procurement decisions. Purchasers in this region frequently require devices that meet cross-border certification standards, favor instruments with long-term service agreements, and value vendors that can demonstrate environmental durability and interoperability with regional data infrastructures. In parallel, some markets within this region are investing in capacity for domestic calibration laboratories and supply chain localization to reduce dependency on distant suppliers.
The Asia-Pacific region exhibits rapid deployment of portable monitoring solutions to support industrial expansion and environmental surveillance, alongside significant investments in nuclear infrastructure planning. Diverse regulatory maturities within the region result in a bifurcated demand pattern: advanced markets adopt cutting-edge semiconductor detectors integrated with analytics platforms, while emerging markets prioritize cost-effective, robust instruments and accessible maintenance services. Cross-region partnerships and technology licensing continue to be important mechanisms for knowledge transfer and local capability building.
Why market leaders are investing in modular detector platforms, embedded analytics, and lifecycle service models to create defensible positions and strengthen customer retention across sectors
Leading companies in the instrumentation space are aligning engineering roadmaps with service-oriented commercial models to deepen customer relationships and capture lifecycle value. Rather than competing solely on device specifications, successful firms are bundling calibration services, extended warranties, and software subscriptions that simplify regulatory compliance and reduce operational friction for end users. Strategic partnerships with semiconductor foundries, analytics providers, and calibration laboratories enable these companies to accelerate time-to-market for new detector platforms while maintaining traceability and auditability across the device lifecycle.Innovative entrants and established manufacturers alike are investing selectively in diamond and silicon detector technologies and in developing modular product families that allow customers to upgrade sensing modules without replacing the entire instrument. This modularity supports longer asset lifecycles and eases integration with telemetry systems. Many firms are also pursuing training and turnkey service offerings targeted at hospital physics departments, industrial safety teams, and research labs, acknowledging that after-sales support and documented calibration histories are often decisive procurement factors.
Finally, corporate strategies increasingly emphasize resilient supply chains, clear documentation for regulatory clearance, and flexible manufacturing footprints that can respond to tariff shifts or sudden demand spikes. These priorities favor companies that can combine engineering depth with operational excellence and strong channel relationships across diverse end-user segments.
High-impact strategic moves for manufacturers and procurement leaders to accelerate semiconductor adoption, operationalize modularity, and secure regulatory-compliant service-led revenue streams
Industry leaders should pursue a coordinated set of actions to convert insight into competitive advantage and to de-risk operations across technology, procurement, and regulatory dimensions. First, prioritize R&D investments in semiconductor-based sensing-both diamond and silicon variants-while maintaining support for ionization chambers and scintillation technologies where they remain the best technical fit for specific applications. This balanced approach preserves market coverage while enabling migration toward devices that offer superior integration and long-term stability.Second, redesign product architectures with modular upgrade paths that allow detectors, readout electronics, and communications modules to be serviced or replaced independently. Such modularity reduces total cost of ownership for buyers and shortens qualification cycles for suppliers. Third, accelerate the development of secure, audit-ready data systems and calibration traceability, as these features are increasingly required by regulators and procurement offices. Fourth, diversify supplier networks and consider nearshoring critical component production to mitigate tariff exposure and ensure predictable lead times.
Fifth, augment hardware offerings with service contracts, remote calibration capabilities, and training programs tailored to hospitals, industrial facilities, nuclear stations, and research institutes. Finally, engage proactively with regulatory bodies and standards organizations to shape certification pathways that recognize new detector technologies, thereby smoothing adoption hurdles and aligning technical innovation with compliance requirements.
A rigorous mixed-methods approach blending stakeholder interviews, laboratory benchmarking, standards analysis, and scenario planning to ensure findings are technically grounded and operationally relevant
The research behind this analysis combined qualitative and structured approaches to deliver robust, actionable conclusions. Primary interviews were conducted with a cross-section of stakeholders including device engineers, calibration laboratory leads, hospital medical physicists, industrial safety officers, and procurement specialists to capture end-user requirements, pain points, and adoption barriers. Complementary expert panels and technical workshops provided deep dives into detector physics, signal processing, and integration challenges, enabling cross-validation of perspectives and identification of common engineering priorities.Secondary efforts incorporated a systematic review of publicly available regulatory guidance, standards documentation, patent filings, and product specification sheets to assess technology trajectories and compliance constraints. Device benchmarking and laboratory tests were used to compare energy response, environmental resilience, and calibration stability across detector classes, while supply chain mapping identified concentration risks for critical components. The methodology emphasized triangulation: qualitative insights were validated against technical measurements and regulatory documentation to ensure consistency. Finally, scenario analysis was used to explore the operational impacts of tariffs, supply disruptions, and regulatory tightening, yielding the strategic recommendations presented earlier.
A concise synthesis emphasizing that success will require coupling detector innovation with traceable calibration, modular design, resilient supply chains, and service-oriented commercialization
The cumulative analysis underscores that the next phase of maturation for ionizing radiation precision instruments will be defined by integration, traceability, and pragmatic engineering choices that reconcile legacy needs with emergent capabilities. Detector innovation-especially within semiconductor domains-offers pathways to improved sensitivity, smaller form factors, and enhanced durability, but the broader value proposition depends on how manufacturers pair hardware with reliable calibration, software-enabled analytics, and service commitments that meet regulatory scrutiny.Procurement strategies that emphasize total cost of ownership, modular upgradeability, and documented calibration histories will favor suppliers that can demonstrate stable supply chains and clear plans for tariff mitigation. Regional differences in regulatory frameworks and adoption readiness will continue to create opportunities for targeted go-to-market strategies, while collaborative approaches between vendors, calibration laboratories, and standards bodies will be essential to accelerate safe deployment. In short, success will accrue to organizations that align technical innovation with operational reliability, deliver traceable data, and provide the services necessary to translate measurement capability into actionable insights for clinical, industrial, and research applications.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Ionizing Radiation Precision Instruments Market
Companies Mentioned
- AMETEK, Inc.
- Arrow-Tech, Inc.
- Atomtex SPE
- Berthold Technologies GmbH & Co. KG
- Biodex Medical Systems, Inc.
- Centronic Ltd. by Exosens
- Fortive Corporation
- Fuji Electric Co., Ltd.
- Honeywell International Inc.
- LND, Inc.
- Ludlum Measurements, Inc.
- Mirion Technologies, Inc.
- Overhoff Technology Corporation
- Polimaster Holdings Ltd.
- Protec GmbH & Co. KG
- PTW Freiburg GmbH
- Radiation Detection Company
- Rotem Industries Ltd.
- S.E. International, Inc.
- Saphymo GmbH by Bertin Technologies
- Thermo Fisher Scientific Inc.
- Tracerco Limited by Johnson Matthey PLC
- X-Z LAB Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
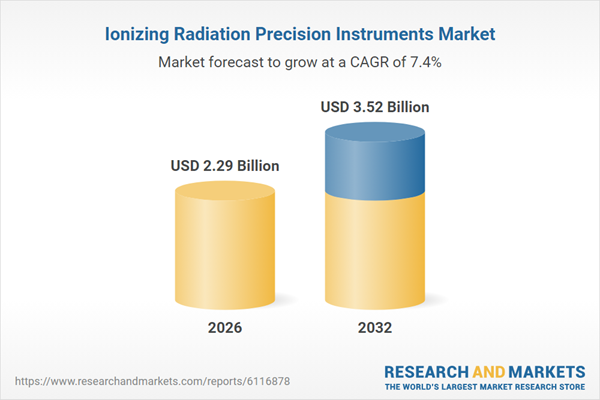
| Estimated Market Value ( USD | $ 2.29 Billion |
| Forecasted Market Value ( USD | $ 3.52 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |