Speak directly to the analyst to clarify any post sales queries you may have.
Positioning Water for Injection systems as critical infrastructure in regulated manufacturing and clinical operations to guide strategic procurement and risk planning
Water for Injection (WFI) systems represent a foundational component of sterile manufacturing, parenteral formulation, and diagnostic workflows where water quality underpins product safety and regulatory compliance. Executives and technical leaders view WFI capability as both a technical asset and a strategic lever: it affects facility design, validation timelines, operating expenditure profiles, and supply chain resilience. As regulatory scrutiny intensifies, the intersection of process reliability, microbial control, and distribution integrity becomes central to sustaining production continuity and protecting patient safety.This introduction frames the WFI landscape from the dual perspectives of operational execution and strategic planning. It outlines the technical modalities of production and distribution while emphasizing the governance frameworks that shape procurement, qualification, and maintenance decisions. For stakeholders charged with capital investments and operational continuity, a nuanced understanding of system architectures, lifecycle maintenance, and the trade-offs between onsite generation versus offsite supply is essential.
Throughout this summary, the goal is to provide a coherent narrative that links process choice, regulatory expectation, and commercial dynamics. By doing so, leaders can align engineering choices with business objectives, mitigate validation risk, and prioritize investments that yield measurable improvements in reliability, compliance, and total cost of ownership over the asset lifecycle.
Emerging technological, regulatory, and supply chain shifts reshaping Water for Injection ecosystems and driving new validation, sustainability, and modularization strategies
The Water for Injection ecosystem is undergoing a period of rapid transformation driven by advances in purification technology, heightened regulatory expectations, and evolving sustainability imperatives. Technological evolution is visible in the maturation of membrane processes, modularization of system components, and adoption of instrumentation that improves real-time monitoring and predictive maintenance capability. These shifts reduce manual intervention and bolster reproducibility across production batches, while enabling more compact and standardized system footprints suitable for modular facilities.Regulatory and quality expectations are also in flux, leading organizations to reevaluate validation strategies and lifecycle management approaches. The emphasis has moved from episodic qualification events to continuous assurance frameworks that leverage in-line analytics and robust data integrity practices. Consequently, organizations are prioritizing system designs that facilitate traceability, support electronic records, and integrate seamlessly with manufacturing execution systems.
Supply chain dynamics are another driving force behind transformative change. Organizations are responding to procurement volatility by diversifying supplier bases, considering alternative material compositions that meet performance specifications, and exploring service models that reduce ownership complexity. Sustainability considerations are prompting investments in energy-efficient distillation options, water recovery strategies, and minimized chemical usage during sanitization. Taken together, these shifts are recasting WFI from a static utility to a strategic asset that influences agility, compliance posture, and environmental stewardship.
Comprehensive assessment of cumulative United States tariff impacts through 2025 on Water for Injection supply chains, capital equipment costs, and sourcing realignment decisions
Recent tariff actions originating from the United States have exerted a tangible influence on the procurement and supply configurations for Water for Injection capital equipment and spare parts. The imposition of elevated import duties on key components raises the landed cost of turnkey systems and specialized instrumentation, which in turn affects procurement timing, vendor selection, and budgetary allocations. Organizations with global procurement footprints have responded by reassessing sourcing geographies and seeking tariff-exempt alternatives where technically feasible.Beyond direct cost effects, tariffs precipitate secondary operational consequences. Extended lead times emerge as suppliers shift production to tariff-favored jurisdictions or reallocate constrained component inventories, creating scheduling challenges for installation and qualification activities. As a result, engineering teams are increasingly incorporating buffer timelines into project plans and exploring phased commissioning approaches to mitigate delay risk. Additionally, higher import costs incentivize local assembly or regional manufacturing partnerships, which can reduce exposure to future tariff volatility but require rigorous supplier qualification and supply chain oversight.
Strategic responses have also included renegotiating service and maintenance agreements to lock in parts availability and adopting modular procurement that allows substitution of non-differentiating components sourced regionally. From a governance perspective, procurement and compliance functions are collaborating more closely to evaluate tariff implications against validation risk, ensuring that sourcing adjustments do not compromise regulatory expectations or system performance. Ultimately, the cumulative tariff environment through 2025 is prompting a rebalancing of cost, resilience, and compliance priorities within WFI programs.
In-depth segmentation insights synthesizing end users, processes, products, system types, applications and production models to inform targeted investment and development choices
A granular understanding of segmentation enables more targeted product development, validation planning, and go-to-market positioning across the Water for Injection landscape. When categorized by end user, the market spans Biotech Firms, Contract Research Organizations, Hospitals, and Pharmaceutical Companies, each of which imposes distinct performance expectations and procurement cadences. Biotech firms and contract research organizations often require flexible, small-scale systems that support rapid development cycles, whereas hospitals prioritize reliability and ease of service for clinical support applications, and pharmaceutical companies focus on scalable systems that integrate with high-volume parenteral manufacturing lines.Process segmentation illuminates the technical trade-offs among distillation, reverse osmosis and electrodeionization, and ultrafiltration. Distillation architectures are differentiated between batch and continuous configurations, with batch systems favored in smaller or legacy installations and continuous distillation preferred for high-throughput operations demanding steady-state control. Reverse osmosis and electrodeionization pathways are analyzed as multi-stage or two-stage designs, where the selection influences recovery rates, pretreatment requirements, and footprint. Ultrafiltration choices encompass hollow fiber and spiral wound membrane formats, each offering distinct flux, cleaning, and retention characteristics that impact system validation and operational procedures.
Product segmentation differentiates cleaning systems, generators, and storage and distribution systems, underscoring the interdependence of sterilization efficacy, production consistency, and hygienic transfer. The dichotomy of system type between multi-use systems and single-use systems raises questions about lifecycle management, cross-contamination risk, and capital allocation strategies. Application segmentation covers diagnostic applications, formulation, and parenteral manufacturing, which dictate purity tolerances, distribution integrity, and sampling regimes. Finally, production mode-whether offsite supply or onsite generation-affects facility design, qualification scope, and operational staffing. Synthesizing these segmentation axes reveals opportunities to tailor system architectures and service offerings to specific user needs, while acknowledging the validation and regulatory frameworks that constrain rapid configuration changes.
Comparative regional analysis highlighting demand drivers, regulatory contrasts, and infrastructure maturity across Americas, Europe, Middle East & Africa and Asia-Pacific markets
Regional dynamics shape investment priorities, regulatory compliance approaches, and supply chain strategies for Water for Injection solutions. In the Americas, demand is influenced by a mature pharmaceutical manufacturing base, an emphasis on continuous improvement, and a regulatory environment that prioritizes product quality and patient safety. Organizations in this region often pursue innovations that reduce operational complexity and support large-scale parenteral production, and they focus on supplier relationships that can deliver integrated validation support and rapid service response.Across Europe, Middle East & Africa, regulatory harmonization and infrastructure heterogeneity coexist, producing differentiated demand. Western European markets emphasize sustainability, energy efficiency, and integration with advanced automation frameworks, while certain markets in the Middle East and Africa are focused on building local capacity and securing dependable supply chains. In these regions, local regulatory expectations and import dynamics influence whether firms opt for onsite generation or offsite supply models, and regional suppliers with localized service networks can play a decisive role.
Asia-Pacific exhibits dynamic growth driven by expanding biomanufacturing, increasing R&D activity, and rising healthcare infrastructure investment. This region balances rapid capacity expansion with a strong focus on cost efficiency, leading to varied adoption paths that include both compact, modular WFI systems for small-scale facilities and high-capacity continuous solutions for export-oriented production. Across all regions, regulatory alignment, service ecosystem maturity, and logistical connectivity form the core determinants of supplier selection and system architecture decisions.
Competitive dynamics and supplier positioning analysis revealing strategic partnerships, M&A pressures, and technology differentiation among major system providers and integrators
Competitive dynamics among suppliers reflect a mix of broad-based system providers, specialist component manufacturers, and independent system integrators. Market leaders differentiate through integrated engineering capabilities, robust after-sales service networks, and comprehensive validation support that aligns with regulatory expectations. Meanwhile, niche players focus on specific modules-such as membrane technologies, distribution loop components, or advanced monitoring systems-offering targeted innovations that can be integrated into larger WFI architectures.Strategic partnerships and channel relationships are increasingly important as buyers seek vendors that can offer end-to-end solutions, including design, installation, qualification, and lifecycle support. Suppliers that invest in digital toolchains for asset management and remote diagnostics secure a competitive advantage by enabling predictive maintenance and reducing unplanned downtime. Additionally, firms that demonstrate strong technical advisory capabilities, with subject matter expertise in sanitization cycles, bioburden control, and distribution engineering, are preferred by organizations undertaking complex validation programs.
Supply-side pressures have elevated the importance of manufacturing flexibility and regional responsiveness. Companies that maintain modular product portfolios and localized assembly or service hubs better navigate tariff-driven cost variability and logistics disruptions. Finally, intellectual property around low-energy distillation designs, high-recovery membrane trains, and validated single-use distribution components remains a differentiator that shapes long-term supplier positioning and buyer preference.
High-impact strategic recommendations for industry leaders to accelerate resilience, optimize total cost of ownership, and futureproof Water for Injection operations
Leaders in manufacturing and clinical production must adopt practical, high-impact measures to strengthen WFI reliability and operational resilience. First, align procurement strategy with validation risk by prioritizing suppliers that provide validated design documentation, comprehensive FAT/SAT protocols, and lifecycle maintenance plans. This reduces commissioning uncertainty and streamlines regulatory submissions. Next, integrate real-time analytics and remote monitoring early in specification development to shift from reactive maintenance to predictive models that increase uptime and reduce emergency service calls.Sustainability and energy efficiency should be core criteria in capital investment decisions. Evaluating options that enable heat recovery, higher recovery rates, and optimized sanitization cycles yields long-term reductions in energy and water consumption. Additionally, adopt a modular philosophy for both capital and spare parts procurement to enable phased commissioning and rapid reconfiguration when production requirements change. This approach enhances flexibility while limiting the validation impact of incremental upgrades.
Finally, strengthen supplier governance by establishing regional service agreements and dual-source strategies for critical components. Coordinate cross-functional teams-procurement, validation, engineering, and quality-to assess tariff exposure, lead-time risk, and supplier capacity constraints. Investing in these practices reduces program delays and positions organizations to respond decisively to regulatory shifts or supply chain disruptions.
Robust research methodology combining primary stakeholder interviews, technical validation, and multi-layered data triangulation to ensure rigorous market intelligence
The research underpinning this executive summary relied on a multi-method approach designed to combine technical validation with stakeholder perspectives. Primary inputs included interviews with engineering leads, quality assurance managers, and procurement officers involved in WFI projects, complemented by discussions with system integrators and component suppliers. These engagements provided direct insight into validation timelines, preferred architectures, and maintenance realities that shape procurement decisions.Technical assessment included review of product specifications, factory acceptance test documentation, and system schematics to evaluate the operational trade-offs among distillation variants, membrane trains, and distribution loop designs. Data triangulation drew on publicly available regulatory guidance and standards relevant to sterile water production and parenteral manufacturing, alongside anonymized case studies of recent installations to surface common failure modes and successful mitigation strategies.
Finally, the analysis applied a synthesis layer that integrated qualitative insights with technical evaluation to produce actionable recommendations. This method ensures that conclusions are grounded in operational realities, validated against technical documentation, and tempered by stakeholder experience, yielding intelligence that supports practical decision making for capital projects and operational improvements.
Conclusive synthesis of systemic trends, operational imperatives, and strategic pathways for organizations deploying Water for Injection across regulated environments
In summary, Water for Injection systems occupy a strategic position at the intersection of quality assurance, operational efficiency, and capital planning. The evolving landscape-characterized by technological advancement, regulatory evolution, and supply chain recalibration-requires organizations to adopt integrated strategies that balance validation rigor with operational flexibility. Process selection, product architecture, and production mode should be chosen with an eye toward lifecycle implications and the organization’s capacity for maintenance and quality governance.Regional nuances and tariff dynamics further complicate sourcing decisions, making supplier selection and service model design critical to program success. Companies that invest in modular designs, digital monitoring, and validated supplier partnerships will be better equipped to maintain continuity, accelerate time to market, and manage total cost of ownership. Ultimately, the most effective strategies are those that integrate cross-functional planning, embrace technological enhancements that reduce manual risk, and maintain strict alignment with regulatory expectations.
This conclusion encourages decision makers to prioritize resilience and validation robustness while remaining open to innovations that improve efficiency and sustainability. The path forward lies in adopting pragmatic, evidence-based approaches that deliver reliable WFI performance and support evolving manufacturing objectives.
Table of Contents
19. ResearchStatistics
20. ResearchContacts
21. ResearchArticles
22. Appendix
Companies Mentioned
- Aqua-Chem, Inc.
- Aquatech International LLC
- Asahi Kasei Corporation
- BWT Holding GmbH
- Calgon Carbon Corporation
- DuPont de Nemours
- Ecolab Inc.
- Evoqua Water Technologies LLC
- GEA Group
- H2O GmbH
- Kurita Water Industries
- MECO Incorporated
- Merck KGaA
- Pall Corporation
- Paul Mueller Company
- Pentair plc
- Sartorius AG
- STERIS plc
- Stilmas S.P.A.
- SUEZ Water Technologies & Solutions
- Suncombe Limited
- Syntegon Technology GmbH
- Thermo Fisher Scientific Inc.
- Veolia Water Technologies
- Xylem Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
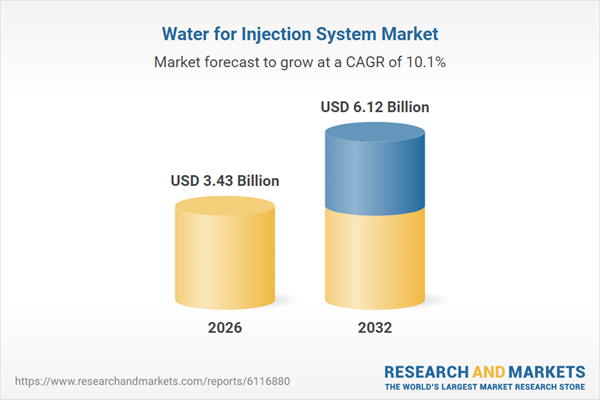
| Estimated Market Value ( USD | $ 3.43 Billion |
| Forecasted Market Value ( USD | $ 6.12 Billion |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |