Speak directly to the analyst to clarify any post sales queries you may have.
Concise industry framing that synthesizes scientific advances, evolving consumer expectations, and regulatory dynamics shaping microbiome-focused cosmetic innovation
The expanding convergence of microbiology, dermatology, and cosmetic formulation is reshaping how product developers and brand strategists approach skin and scalp health. Advances in microbiome science have shifted the conversation from purely external aesthetics to an integrated understanding of host-microbe interactions and how those interactions influence barrier function, inflammation, and long-term skin resilience. Consequently, companies are adapting research pipelines, ingredient sourcing strategies, and regulatory monitoring to incorporate microbiome-friendly claims and evidence-backed functional benefits.Alongside scientific progress, consumer expectations have evolved in ways that place transparency, efficacy and personalization at the center of purchase decisions. Shoppers now seek products that promise gentle modulation of the skin ecosystem rather than indiscriminate eradication of microbes. This behavioral change has compelled formulators to prioritize gentle surfactants, targeted prebiotic and postbiotic actives, and delivery systems that support microbial balance. In parallel, influencers and clinical practitioners are amplifying awareness of microbiome concepts, which increases demand for educational content and substantiated claims.
Moreover, the regulatory and labeling environment is gradually catching up with innovation, prompting companies to invest in robust safety assessments and clinical endpoints that resonate with regulators and healthcare professionals. Taken together, these dynamics are redefining R&D priorities, commercial positioning, and cross-functional collaborations across the value chain, creating both challenges and opportunities for incumbents and entrants alike.
Overview of structural and technological shifts driving microbiome cosmetics toward evidence-based formulation, resilient supply chains, and differentiated consumer messaging
The landscape for microbiome-directed cosmetics is experiencing transformative shifts as technological maturation intersects with changing market behaviors and value chain realignment. Cutting-edge analytical tools and in vitro models are enabling deeper characterization of microbial communities and host responses, which in turn allows for more precise ingredient design and targeted formulations. As a result, research teams are moving from descriptive microbiome profiling toward mechanistic testing and validated functional claims that demonstrate benefits such as barrier repair and sensitivity reduction.Concurrently, manufacturing and supply chain strategies are evolving to support stability and traceability of biologically derived ingredients, prompting a reassessment of raw material sourcing and cold-chain logistics when necessary. This operational transformation is mirrored by retail and distribution channels adjusting their offer sets to accommodate a growing spectrum of microbiome-friendly SKUs across body, face, and hair care. Partnerships between ingredient specialists, clinical laboratories, and brand houses are becoming more common, facilitating the translation of scientific insights into commercially viable products.
Consumer communication is also shifting from broad wellness narratives to more nuanced, education-driven storytelling that emphasizes evidence, safety, and personalization. Brands that successfully integrate these elements are differentiating on credibility and long-term consumer trust. Ultimately, the cumulative effect of these shifts is a market that prizes demonstrable science, supply chain robustness, and transparent messaging, demanding strategic adaptability from businesses across the ecosystem.
Assessment of tariff-driven operational responses and strategic shifts influencing supplier choices, formulation priorities, and go-to-market timing in the United States
The policy landscape in the United States has introduced tariff adjustments that reverberate across ingredient procurement, finished-goods importation, and manufacturing strategies. These changes have prompted procurement teams and senior leadership to reassess supplier diversity, nearshoring viability, and inventory management practices. Companies dependent on imported specialty actives and excipients have faced rising landed costs, which has led some to accelerate initiatives aimed at securing local suppliers or reformulating to reduce reliance on tariff-impacted inputs.Manufacturers have responded by optimizing production footprints and implementing flexible sourcing frameworks, thereby reducing exposure to any single trade corridor. Additionally, product development teams are prioritizing ingredient efficiency and multifunctionality to preserve margins while maintaining performance profiles that meet consumer expectations. Regulatory compliance costs and extended lead times for custom ingredients have also influenced go-to-market timing, encouraging portfolio rationalization and phased launches to mitigate cashflow pressures.
From a commercial perspective, retailers and brands are re-evaluating pricing architecture, promotional cadence, and channel-level economics to protect consumer accessibility without compromising brand positioning. In short, the tariff environment has catalyzed operational resilience efforts, shifted strategic supplier relationships, and incentivized greater vertical coordination between procurement, R&D, and commercial functions.
In-depth segmentation perspectives illuminating product innovation choices, distribution channel dynamics, and ingredient-driven application strategies across consumer cohorts
Segment-level analysis reveals diverse strategic implications for product design, distribution, and consumer targeting. Across product types, body care portfolios extend beyond wash and lotion formats to include serums aimed at delivering targeted microbiome-supportive actives; face care strategies emphasize creams, lotions, masks, and serums with day and night cream sub-differentiation to accommodate circadian and barrier-repair claims; hair care innovation is expanding through conditioners, masks, and shampoos formulated to balance scalp microbiota and improve follicle health. Distribution dynamics show an ongoing duality between offline footprints-where pharmacies, retail stores and specialty clinics offer clinical credibility and trial opportunities-and online ecosystems, where brand websites and ecommerce marketplaces provide rich data capture and personalized marketing.Ingredient segmentation underscores distinct formulation pathways: postbiotics are being leveraged for barrier restoration and anti-inflammatory effects, prebiotics for selective nourishment of beneficial commensals, probiotics in stabilized topical formats where feasible, and synbiotics that combine approaches for synergistic outcomes. Application-focused R&D is concentrating on acne treatment, anti-aging benefits with a split focus on firming and wrinkle reduction, barrier repair, moisturizing functionality, and sensitivity soothing. Form choices such as creams, lotions, masks, powders, and serums are being selected based on delivery efficacy and consumer preferences. End-user strategies address differentiated positioning for men, women, and unisex offerings while age-focused segmentation tailors efficacy and sensory profiles for adults, elderly consumers, and teens. Finally, price-tier strategies span mass through ultra-premium positioning, with premiumization tactics emphasizing clinically validated actives and elevated sensory experiences to justify higher price points.
Regional dynamics that inform market entry priorities, localized formulation strategies, and regulatory planning across the Americas, Europe Middle East and Africa, and Asia Pacific
Regional dynamics are shaping strategic priorities for product formulation, regulatory planning, and market entry sequencing. In the Americas, there is a strong emphasis on clinically validated benefits and clean-label narratives, with brands often combining science-led communication with broad retail distribution to drive mainstream adoption. Europe, Middle East & Africa present a heterogeneous regulatory and consumer environment where local ingredient approvals, cultural skincare routines, and sustainability expectations require tailored formulations and localized messaging. In Asia-Pacific, rapid consumer adoption of novel cosmetic science and high receptivity to premiumization are driving accelerated product innovation and shorter product life cycles; brands in this region frequently lead in adopting new textures, delivery systems, and hybrid wellness-beauty propositions.Each region exerts influence on ingredient sourcing decisions, laboratory validation requirements, and partnership strategies. For multinational brands, regional regulatory variance necessitates adaptable label claims and modular clinical protocols that can be scaled or adjusted to local requirements. Conversely, local and regional players often capitalize on cultural insights, faster innovation loops, and agile go-to-market execution to capture share in high-growth categories. Together, these regional contrasts inform prioritization of market entry, investment in localized clinical studies, and channel strategies to align with consumer expectations and regulatory realities.
Competitive landscape insights highlighting ingredient innovation, collaborative clinical validation, and strategic partnerships that drive differentiation and commercial advantage
Competitive activity within the microbiome cosmetics sphere is marked by a mix of established beauty houses, nimble indie brands, and specialized ingredient suppliers collaborating to translate microbiome science into marketable products. Ingredient innovators are differentiating by developing stabilized postbiotic complexes and proprietary prebiotic blends that can be substantiated through in vitro and clinical evidence. Meanwhile, consumer brands are pairing these ingredient solutions with elevated sensory profiles and targeted educational content to build trust and shorten the path to purchase.Strategic partnerships between clinical research labs and brand teams are becoming standard practice, enabling claims to be supported by reproducible data while meeting regulatory expectations. Additionally, contract manufacturers and boutique formulation studios are offering modular services that allow brands to iterate rapidly across texture, efficacy, and claim sets. From a go-to-market perspective, alliances with dermatologists, trichologists, and specialty clinics help validate product positioning and open clinical distribution channels. Investment and M&A activity tend to focus on acquiring unique ingredient IP, expanding microbiome testing capabilities, or securing talent with specialized formulation expertise. As a result, competitive advantage increasingly rests on the integration of proprietary actives, robust clinical evidence, and compelling consumer narratives.
Action-oriented strategic measures for industry leaders to strengthen scientific credibility, operational resilience, and multi-channel commercial execution
Leaders pursuing durable growth should prioritize an integrated strategy that aligns R&D, procurement, and commercial execution. First, invest in robust evidence generation that pairs mechanistic laboratory studies with consumer-relevant clinical endpoints to substantiate efficacy and support sensible claims. Second, diversify supply chains and explore regional supplier partnerships to mitigate tariff exposure and ensure continuity for specialty actives. Third, adopt modular formulation frameworks that enable rapid iteration across formats-creams, lotions, masks, serums-and that reconcile sensory excellence with microbiome-friendly performance.Fourth, refine channel strategies by balancing the credibility and tactile trial benefits of offline channels with the data-driven personalization and scale of online platforms. Fifth, build educational marketing that elevates consumer understanding of microbiome concepts while anchoring messages in safety and results; this will reduce skepticism and accelerate adoption. Sixth, pursue strategic collaborations with clinical experts and select ingredient partners to co-develop substantiated portfolios and to accelerate time-to-market. Finally, implement price-tiered offerings that allow brands to capture mass-market consumers while testing premium propositions reserved for clinically substantiated or high-sensory products. Collectively, these actions will strengthen resilience, support margin preservation, and enhance the potential for sustained consumer trust and commercial momentum.
Comprehensive multi-method research framework combining expert interviews, laboratory validation assessment, and rigorous secondary synthesis to ensure actionable insights
The research approach blends primary qualitative interviews, laboratory validation review, and systematic secondary analysis to construct a holistic view of the microbiome cosmetics landscape. Primary inputs were gathered through in-depth interviews with formulation scientists, procurement leaders, clinical researchers, and retail strategists to capture operational challenges, adoption barriers, and innovation priorities. Laboratory validation review examined methodologies for assessing microbial modulation, barrier integrity, and biomarkers relevant to claims, focusing on reproducibility and clinical relevance.Secondary analysis synthesized public regulatory guidance, patent activity, product launches, and consumer behavior signals to contextualize primary insights. Triangulation was used to reconcile differences between lab findings and market anecdotes, ensuring that conclusions reflect both technical feasibility and commercial viability. Quality control measures included cross-validation of interview transcripts, methodological transparency for laboratory evidence assessment, and iterative peer review by subject matter experts. Ethical considerations and privacy protections were observed for all primary data collection. This multi-method approach provides a defensible and actionable foundation for strategic decision-making and product development planning.
Concluding synthesis that emphasizes evidence-driven differentiation, supply resilience, and regional sensitivity as the pillars for sustained success in microbiome cosmetics
In conclusion, the intersection of advancing microbiome science and shifting consumer expectations is redefining priorities across formulation, supply chain, and commercial strategy. Brands that marry credible evidence with compelling consumer experiences, while maintaining operational agility, will be best positioned to capture the long-term benefit of this evolution. Operational risks such as tariff volatility and supply concentration can be mitigated through deliberate procurement diversification and modular formulation design, preserving both margin and time-to-market.Looking ahead, the companies that invest early in scalable clinical validation, transparent communication, and partnerships with ingredient innovators and clinical experts will establish durable differentiation. At the same time, regional nuances and channel dynamics require tailored approaches that respect local regulatory frameworks and consumer rituals. By integrating the strategic recommendations of this report-focused on evidence generation, supply resilience, and clear consumer education-industry stakeholders can navigate complexity and convert scientific opportunity into commercially meaningful outcomes.
Table of Contents
21. ResearchStatistics
22. ResearchContacts
23. ResearchArticles
24. Appendix
Companies Mentioned
- Amorepacific Corporation
- Aurelia Skincare Ltd.
- Beiersdorf AG
- Biomilk Skincare Ltd.
- DSM-Firmenich AG
- Estée Lauder Companies Inc.
- Gallinée Ltd.
- Givaudan SA
- Glowbiotics LLC
- Johnson & Johnson Services, Inc.
- L’Oréal S.A.
- Mother Dirt, LLC
- Procter & Gamble Company
- Shiseido Company, Limited
- Solabia Group
- Symrise AG
- Unilever PLC
- Venn Skincare, Inc.
- Yun Probiotherapy NV
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
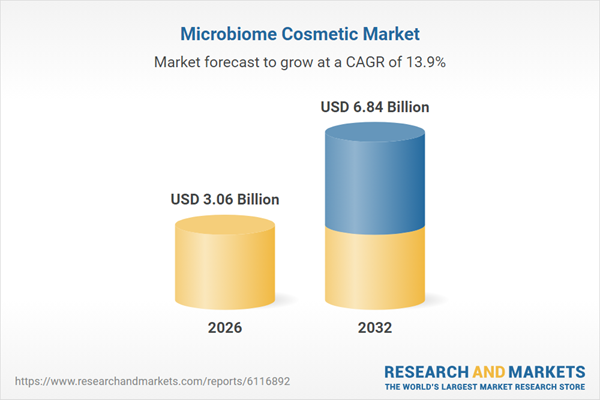
| Estimated Market Value ( USD | $ 3.06 Billion |
| Forecasted Market Value ( USD | $ 6.84 Billion |
| Compound Annual Growth Rate | 13.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |