Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive orientation to sulfamethoxazole and sodium salt that frames clinical importance, formulation considerations, regulatory pressures, and supply chain dynamics
Sulfamethoxazole and its sodium salt have remained central to antibacterial therapy for decades, combining established clinical utility with evolving commercial and manufacturing dynamics. The introduction below situates these active pharmaceutical ingredients within the broader context of antimicrobial stewardship, regulatory oversight, and pharmaceutical supply chain transformation. It highlights the molecular and therapeutic characteristics that sustain clinical demand while framing the contemporary drivers that influence production, distribution, and commercialization strategies.The compound’s pharmacological profile underpins its continued use in combination regimens and monotherapy applications across human and veterinary medicine. As treatment protocols evolve due to resistance patterns and diagnostic improvements, manufacturers and formulators must reconcile clinical efficacy with formulation choices, regulatory pathways, and manufacturing scalability. At the same time, trade policies, raw material sourcing, and contract manufacturing relationships introduce operational variability that affects how producers and purchasers manage inventory and procurement risk.
Consequently, understanding this molecule requires a multidimensional lens that integrates clinical practice, formulation science, regulatory expectations, and commercial logistics. The introduction establishes this integrated perspective so that subsequent analysis can examine how structural shifts in policy, distribution, and end-user preferences interact to shape strategic choices for pharmaceutical companies, healthcare providers, and supply chain partners.
How regulatory tightening, advanced manufacturing practices, and digital distribution channels are reshaping supply reliability and competitive differentiation across the antibiotic landscape
The landscape for sulfamethoxazole and its sodium salt is undergoing transformative shifts driven by regulatory realignment, digital-enabled distribution, and evolving patterns of antimicrobial stewardship. One major inflection point is the increasing emphasis on targeted use of established antibiotics, which has elevated demand for higher-quality formulations and for reliable supply lines that can support stewardship initiatives without introducing shortages.Concurrently, technological and operational changes in manufacturing are reshaping capacity planning and quality assurance. Continuous processing and modular manufacturing approaches are being piloted to improve throughput and batch consistency, while contract development and manufacturing organizations expand capabilities to respond to shorter product life cycles and increased customization. These manufacturing innovations interact with regulatory expectations that are tightening quality oversight for active pharmaceutical ingredients and finished-dose forms, prompting companies to invest in traceability and automated testing to ensure compliance.
On the commercial front, digitization of procurement and the rise of accredited online distribution channels are altering traditional buyer-supplier relationships. As stakeholders increasingly rely on electronic tendering and e-procurement systems, rapid responsiveness, validated supply chains, and transparent compliance records become competitive differentiators. Taken together, these shifts are creating new strategic imperatives: firms must invest in resilient manufacturing, robust quality systems, and digitally enabled commercial capabilities to remain competitive in a market where reliability and compliance matter as much as price.
Evaluating how United States tariff adjustments and related policy shifts have influenced supply chain sourcing choices, inventory strategies, and production economics for pharmaceutical stakeholders
The cumulative impact of recent tariff actions and trade policy adjustments in the United States is exerting pressure across the pharmaceutical value chain, with particular implications for sulfamethoxazole and its sodium salt. Tariff-induced cost increases on raw materials and intermediate chemical inputs have amplified procurement complexity for manufacturers that rely on global supplier networks. As import duties elevate landed costs, firms face a choice between absorbing margin compression, passing costs downstream, or restructuring sourcing to mitigate exposure.In response, several manufacturers have adjusted procurement strategies by diversifying supplier bases and increasing onshore buffer stocks to smooth supply variability. These adjustments have implications for working capital and warehouse capacity, and they have prompted reassessments of vendor qualification timelines to maintain compliant supply. For companies pursuing vertical integration, tariffs have accelerated interest in localized upstream production for critical intermediates, which can reduce import dependency but necessitates capital investment and time for regulatory qualification.
Downstream, distributors and healthcare providers are recalibrating inventory management and contractual terms to remain agile in the face of price volatility. Hospitals and clinics are engaging in scenario planning to ensure continuity of care during supply disruptions, while veterinary supply chains are similarly adjusting procurement cadence. Policymakers and industry groups are monitoring these dynamics and exploring targeted relief or trade offsets to preserve access to essential medicines, underscoring the interplay between trade policy and public health imperatives.
Deep segmentation-driven perspectives that align application, type, dosage form, distribution channel, and end-user distinctions to inform targeted product and commercial strategies
Segmentation analysis reveals nuanced demand drivers and development pathways across therapeutic applications, product types, dosage forms, distribution pathways, and end-user settings. When viewed through the lens of application, the market encompasses both human and veterinary uses, with veterinary demand subdivided into companion and livestock segments that each impose distinct formulation and regulatory requirements. Human applications tend to prioritize regulatory-compliant finished-dose forms suitable for hospital use and outpatient prescriptions, whereas veterinary applications often require flexible packaging and dosing suited to animal care settings.Type-based segmentation differentiates sodium salt and sulfamethoxazole, and that distinction informs formulation strategy and stability management during manufacturing and distribution. Dosage form segmentation further clarifies opportunities and constraints: capsules, which may be hard or soft, often serve outpatient and consumer-preference segments; tablets, available as film-coated or scored, support dose flexibility and extended shelf life; suspensions and intravenous forms address pediatric, hospitalized, and acute-care scenarios where rapid titration or parenteral administration is required.
Distribution channel dynamics influence commercial tactics, with hospital pharmacies, online pharmacies-both registered and unregistered-and retail pharmacies, comprising chain and independent outlets, each demanding tailored logistics, compliance documentation, and commercial support. End-user segmentation spans clinics, home healthcare, hospitals, and specialty centers; clinics split into inpatient and outpatient settings while hospitals separate into private and public operations, each of which presents different purchasing behaviors, approval processes, and budgetary constraints. Integrating these segmentation layers enables more precise positioning of molecules, formulations, and services to match clinical requirements and stakeholder procurement practices.
Regional strategic imperatives that reconcile regulatory diversity, channel structures, and supply chain complexity across the Americas, Europe Middle East & Africa, and Asia-Pacific
Regional dynamics shape demand patterns, regulatory expectations, and supply-chain priorities for sulfamethoxazole and its sodium salt, and understanding those regional differences is essential for effective market engagement. In the Americas, healthcare systems vary from high-concentration institutional procurement to broader retail networks, driving a need for reliable hospital supply chains and strong retail channel penetration. Regulatory oversight and reimbursement practices in this region encourage manufacturers to emphasize compliance documentation and post-market surveillance data.Across Europe, Middle East & Africa, regulatory frameworks present a mosaic of centralized and country-specific requirements, necessitating adaptable registration strategies and localized pharmacovigilance capabilities. This region places a premium on quality certification and alignment with international standards, while logistics complexity in some geographies increases the importance of robust cold-chain and secure distribution practices. Transitioning to Asia-Pacific, rapid growth in healthcare access, expanding outpatient services, and varied regulatory maturity are shaping both demand and manufacturing opportunities. Manufacturers often pursue regional distribution partnerships and in-country registration pathways to accelerate market entry and address pricing and accessibility objectives.
Taken together, these regional trends underscore the importance of tailoring regulatory, commercial, and supply-chain approaches to local contexts. Companies that build nuanced regional strategies can better navigate regulatory diversity, align production and inventory allocation with demand centers, and optimize partnerships for distribution and manufacturing to meet regional healthcare priorities.
Competitive and operational behaviors among API specialists, generics manufacturers, and CDMOs that determine access, reliability, and differentiation in antibiotic supply chains
Competitive dynamics in the sulfamethoxazole and sodium salt space are characterized by a mix of established generics manufacturers, active pharmaceutical ingredient specialists, contract development and manufacturing organizations, and distributors focused on regulatory-compliant logistics. Firms that prioritize quality systems, regulatory agility, and supply-chain transparency tend to command preferential access to institutional procurement and tenders. Meanwhile, companies that offer flexible formulation and packaging options can capture demand from outpatient and veterinary segments that require differentiated delivery formats.Strategic behaviors observed across the sector include consolidation of upstream suppliers to stabilize procurement, investments in analytical and quality-control capabilities to meet increasingly stringent inspection regimes, and selective investment in capacity for high-priority intermediates. Many players are also layering commercial capabilities, such as digital order management and e-procurement integration, to reduce friction with hospital and retail buyers. Collaboration between API producers and finished-dose manufacturers has intensified to ensure consistency in supply and to accelerate regulatory filings.
Innovation is not solely technological; business model experimentation-such as supply agreements with volume commitments, localized manufacturing hubs, and bundled service offerings that include training and pharmacovigilance support-has emerged as a means to differentiate in a competitive environment. Firms that balance operational excellence with customer-centric commercial models are better positioned to sustain long-term relationships with hospitals, clinics, veterinary distributors, and retail networks.
Actionable strategic moves for manufacturers and distributors to bolster supply resilience, regulatory readiness, and customer-centric commercial models in antibiotic markets
Industry leaders should act decisively to strengthen supply resilience, enhance regulatory readiness, and align commercial models with evolving buyer expectations. Prioritizing dual-source procurement for critical intermediates and finished-dosage components will reduce single-supplier exposure and improve continuity of supply. Concurrently, investing in localized buffer capacity or nearshoring selective production can mitigate tariff and transportation risk while supporting faster regulatory submission timelines for regional markets.On the regulatory and quality front, organizations should escalate investment in automated quality systems, digital batch release, and comprehensive traceability across the supply chain to meet intensifying inspection standards. These capabilities also support faster responses to safety signals and product lifecycle events. Commercially, strengthening relationships with hospital formularies, chain pharmacy groups, and accredited online distributors through value-add services-such as technical training, bundled pharmacovigilance support, and responsive replenishment programs-will create stickiness and reduce churn.
Finally, leadership teams should embed scenario planning into strategic decision-making to anticipate trade disruptions, sudden demand shifts, or regulatory changes. By formalizing cross-functional playbooks that cover procurement contingencies, production re-routing, and prioritized allocations for critical customers, companies can improve responsiveness and preserve clinical access during periods of supply instability.
A transparent multi-source research framework combining regulatory, scientific, trade, and stakeholder insights with supply chain analysis and scenario testing for rigorous validation
The research methodology underpinning this analysis combines a structured review of primary and secondary data sources, triangulation through expert interviews, and iterative validation to ensure robust and defensible conclusions. Secondary research included a systematic examination of regulatory filings, peer-reviewed scientific literature, publicly available trade and customs statistics, clinical guidelines, and product registration databases to establish the factual foundation regarding therapeutic use, manufacturing practices, and regulatory expectations.Primary research comprised in-depth interviews with a cross-section of industry stakeholders, including formulators, API suppliers, quality and regulatory leads, procurement managers at hospitals and veterinary distributors, and logistics providers. These conversations were used to validate emerging themes, interrogate operational constraints, and capture forward-looking perspectives on adoption of new manufacturing practices and distribution models. Analytical methods employed include supply-chain mapping, risk-profile analysis, and scenario planning exercises to assess potential impacts of policy and market shifts.
Throughout the study, findings were subjected to corroboration across data sources to minimize bias and to highlight areas of consensus and divergence. Limitations are acknowledged where data access is constrained by proprietary information or where rapidly evolving policy environments may alter conditions between research collection and publication. Nevertheless, the methodology emphasizes transparency, reproducibility, and practical relevance for decision-makers.
Concluding synthesis of clinical, regulatory, and commercial dynamics that shape strategic priorities for preserving access and ensuring sustainable supply of essential antibiotics
In conclusion, the market environment for sulfamethoxazole and its sodium salt is being reshaped by regulatory tightening, trade policy adjustments, and shifts in manufacturing and distribution practices. These forces are prompting stakeholders to re-evaluate sourcing strategies, invest in quality and traceability systems, and pursue commercial models that emphasize reliability and compliance alongside cost efficiency. The interplay between clinical stewardship priorities and commercial realities underscores the need for integrated strategies that balance patient access with sustainable supply economics.Looking forward, firms that proactively diversify supply bases, adopt advanced manufacturing and quality technologies, and strengthen partnerships across distribution channels will be better positioned to navigate disruptions and capture opportunities. Equally important is the ability to translate technical and regulatory insights into practical commercial programs that support end users in hospitals, clinics, home healthcare, and veterinary settings. By aligning operational capabilities with the evolving expectations of regulators and buyers, organizations can safeguard continuity of care while sustaining competitive performance.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- Abbott Laboratories
- Aurobindo Pharma Limited
- Bayer AG
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Ltd.
- Hetero Drugs Limited
- Lupin Limited
- Macleods Pharmaceuticals Ltd.
- Merck & Co., Inc.
- Mylan N.V.
- Natco Pharma Limited
- Novartis AG
- Pfizer Inc.
- Roche Holding AG
- Sandoz International GmbH
- Sanofi S.A.
- Strides Pharma Science Limited
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Torrent Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
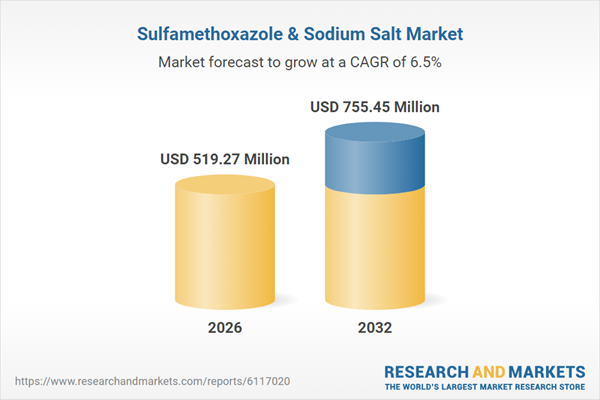
| Estimated Market Value ( USD | $ 519.27 Million |
| Forecasted Market Value ( USD | $ 755.45 Million |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |