Speak directly to the analyst to clarify any post sales queries you may have.
A concise but comprehensive orientation to Vindesine Sulfate API strategic importance across clinical, regulatory, and supply chain decision contexts
Introduction to the contemporary Vindesine Sulfate active pharmaceutical ingredient landscape and strategic relevance for stakeholders
Vindesine Sulfate continues to occupy an important role in oncology therapeutics where microtubule inhibition remains a validated mechanism of action. As clinicians refine treatment regimens and formulary managers reassess procurement pathways, the API’s stability, manufacturability, and supply continuity have emerged as decisive criteria for clinical use and contract negotiations. Stakeholders including clinical trial sponsors, generic manufacturers, and hospital procurement teams require clear, actionable intelligence to align development plans and sourcing strategies with evolving clinical practice.Recent advances in formulation science and shifts in outpatient oncology care delivery have prompted renewed attention to route-specific administration profiles and dosage form preferences. Regulatory agencies are emphasizing manufacturing oversight and pharmacovigilance for cytotoxic agents, creating an environment where regulatory strategy and quality assurance are as influential as traditional cost and access drivers. Consequently, a nuanced view of the API’s role across therapy lines, patient populations, and care settings is crucial for medical affairs, commercial leaders, and supply chain executives.
This report is designed to present a disciplined, evidence-based synthesis of current dynamics that matter most to decision-makers, enabling teams to prioritize investments, optimize contract terms, and mitigate downstream risks associated with formulation transitions or sourcing disruptions.
How evolving clinical priorities, intensified manufacturing expectations, and shifting market access frameworks are redefining Vindesine Sulfate strategy and competitive advantage
Transformative shifts remoulding the Vindesine Sulfate landscape and the forces driving near-term strategic change
The clinical environment for cytotoxic agents is evolving under twin pressures of precision oncology and value-driven care. These pressures have redefined priorities around tolerability, administration convenience, and combination regimen compatibility. As targeted therapies and immuno-oncology agents expand therapeutic options, conventional cytotoxics must be positioned based on clear evidence of incremental clinical benefit or pragmatic advantages in cost and accessibility. This has accelerated reformulation initiatives and selective lifecycle investments where a clear clinical niche remains.Concurrently, manufacturing and regulatory expectations are intensifying. Quality by design principles and tighter scrutiny of sterility and cross-contamination controls in cytotoxic API production are prompting capital investments and supplier consolidation. Such shifts favor manufacturers capable of demonstrating robust quality systems and compliant global footprints. At the same time, digital supply chain visibility and strategic supplier partnerships are becoming essential to manage lead times and buffer against raw-material interruptions.
Market access frameworks are also changing. Payer emphasis on real-world outcomes and total cost of care influences formulary placement and clinician prescribing behavior. These converging forces mean that competitiveness will be determined not only by unit cost but also by demonstrable reliability, formulation versatility, and regulatory readiness.
Assessing the downstream consequences of United States tariff policy shifts in 2025 and practical supply chain responses to maintain continuity and competitive positioning
The cumulative consequences of United States tariff developments in 2025 and how they affect procurement, manufacturing, and supply resilience
Recent tariff actions and trade policy recalibrations in 2025 have introduced additional variables into procurement and manufacturing decision-making for critical APIs. Supply chain planners and procurement leaders are reassessing supplier portfolios to account for potential cost increases, re-routing options, and added compliance requirements. Tariff-related cost pressures can reverberate through contract negotiations, prompting both strategic sourcing shifts and localized manufacturing investments to preserve margin and supply continuity.In reaction, several stakeholders have revisited nearshoring and multi-sourcing strategies to reduce exposure to tariff volatility and to maintain control over quality oversight. These adjustments carry implications for capital allocation, lead times, and regional regulatory interfaces. Importantly, tariff consequences are not uniform across the value chain: raw material inputs, intermediate processing steps, and finished API shipments can each be affected differently, requiring granular cost-to-serve analyses to identify the most effective mitigation levers.
Finally, commercial teams must factor potential tariff impacts into pricing discussions and contracting cadence. Transparent supplier agreements, scenario-based procurement playbooks, and contingency inventory policies are practical steps to manage uncertainty and sustain clinical supply commitments in a tariff-sensitive environment.
Deep segmentation intelligence revealing how administration routes, dosage formats, distribution outlets, and clinical uses converge to shape commercial and clinical strategies
Key segmentation insights that reveal where clinical utility, formulation choices, and service delivery intersect to influence strategic planning
Route of administration splits demand patterns between intramuscular, intravenous, and subcutaneous approaches, with each route exerting distinct requirements on formulation stability, delivery device compatibility, and nursing or outpatient administration protocols. Dosage form differentiation between liquid presentations and lyophilized powder affects cold chain needs, reconstitution workflows, and shelf life considerations, which in turn shape procurement preferences across care settings. Distribution channel dynamics show that hospital pharmacy procurement, online pharmacy platforms, and retail pharmacy availability each present unique regulatory compliance, inventory management, and reimbursement challenges.End user segmentation highlights divergent purchasing criteria across cancer research institutes, clinics, and hospitals; research institutions prioritise batch consistency for study comparability, clinics favour outpatient-friendly presentations, and hospitals emphasise readiness for complex inpatient regimens. Application-level distinctions between combination therapy and single agent therapy influence volume predictability, co-packaging opportunities, and clinical collaboration models. Therapeutic indication focus areas such as leukemia and lymphoma impose specific dosing regimens and safety-monitoring protocols that feed back into labeling and patient education materials. Patient type segmentation, distinguishing adult and pediatric cohorts, drives formulation adjustments, dosing flexibility, and paediatric-specific safety data needs that manufacturers and regulators must address to support broad clinical adoption.
Regional strategic contrasts across the Americas, Europe Middle East & Africa, and Asia-Pacific that dictate differentiated regulatory, manufacturing, and commercial playbooks
Regional insights clarifying divergent dynamics across the Americas, Europe Middle East & Africa, and Asia-Pacific that influence investment and go-to-market choices
The Americas region exhibits mature regulatory structures and integrated payer systems, which place a premium on demonstrable clinical benefit and supply continuity; stakeholders often pursue contract terms that secure long-term inventory reliability and quality assurance. In the Europe, Middle East & Africa block, heterogeneous regulatory regimes and varied reimbursement landscapes demand tailored regulatory strategies and localized evidence packages to facilitate market entry and uptake. Additionally, regional procurement mechanisms and tendering frameworks often influence distribution partnerships and pricing approaches.Asia-Pacific presents a spectrum of opportunities and operational considerations, with several markets emphasizing domestic manufacturing capability and regulatory pathways that can favour local registration efforts. Rapidly expanding oncology care capacity in some markets is increasing demand for established cytotoxic agents while also accelerating interest in cost-effective supply models. Across all regions, regulatory harmonization efforts, regional raw material sourcing practices, and logistics infrastructure differences materially affect how manufacturing footprint decisions and distribution strategies are prioritized.
Competitive and collaborative company behaviours that prioritize manufacturing excellence, regulatory readiness, and targeted innovation to maintain clinical relevance
Company-level competitive dynamics that define how market leaders and challengers are positioning around quality, capacity, and innovation pathways
Leading organisations are concentrating on demonstrating manufacturing excellence, regulatory compliance, and resilient supply chains to meet hospital and clinical demand reliably. Investment in aseptic processing upgrades, validated cold chain logistics, and enhanced quality oversight is a common strategic response to heightened regulatory expectations. Some companies are also pursuing lifecycle management strategies, including reformulation and alternative delivery systems, to extend clinical relevance and create differentiation in procurement evaluations.Challengers and niche players are seeking to carve sustainable roles through specialization, targeted partnerships, and flexible contract manufacturing arrangements that address specific clinical or regional needs. Collaboration between R&D groups and commercial teams is intensifying, with cross-functional programs aimed at aligning clinical evidence generation with payer and provider expectations. Additionally, alliances with distribution partners and service providers are being used to streamline product access across diverse care channels and to reduce time-to-shelf for newly registered presentations.
Practical and prioritized recommendations for leaders to shore up supply resilience, align clinical value propositions, and optimise commercial execution rapidly
Immediately actionable recommendations that industry leaders can implement to strengthen supply resilience, enhance clinical alignment, and unlock commercial opportunities
Reassess supplier portfolios with a focus on dual-sourcing critical intermediates and finished API to reduce single-source exposure while negotiating contractual terms that incentivise reliability. Adopt a tiered inventory strategy that balances cost efficiency with clinical risk mitigation, and formalize contingency playbooks to manage supplier disruptions or regulatory holds. Invest in manufacturing upgrades that align with current regulatory expectations for cytotoxic agents, emphasizing process control, contamination prevention, and end-to-end traceability to support audits and tender evaluations.Enhance clinical engagement by generating real-world evidence and health-economic narratives that clarify the API’s role in contemporary regimens, and align medical affairs activities with key opinion leaders to surface pragmatic advantages that matter to prescribers. Tailor commercial approaches by segmenting customers based on administration route, dosage form needs, and distribution channel preferences to optimise packaging, delivery logistics, and contracting terms. Finally, integrate trade policy scenario planning into procurement and pricing models so that tariff volatility is embedded in decision-making rather than treated as episodic risk.
Transparent and rigorous research methodology combining primary stakeholder interviews, secondary regulatory and clinical evidence, and triangulation for robust validation
Research methodology explaining the rigorous, multi-source approach used to synthesize clinical, regulatory, and commercial intelligence with robust validation steps
The analysis draws on a structured combination of primary and secondary research methods to ensure balanced, verifiable conclusions. Primary research included in-depth interviews with industry practitioners, quality assurance professionals, formulary managers, and clinical experts to capture frontline perspectives on administration preferences, supply challenges, and evidence needs. Secondary research encompassed peer-reviewed literature, regulatory guidance documents, and public filings to corroborate clinical and manufacturing assertions and to map regulatory expectations across jurisdictions.Findings were triangulated using cross-source validation to reconcile differing perspectives and to identify convergent themes. Quality controls included peer review of technical assumptions, validation of regulatory interpretations with subject-matter experts, and consistency checks to ensure clinical descriptions matched current practice. Wherever uncertainty remained, sensitivity of outcomes to alternative assumptions was documented and clearly described so that readers can interpret insights within appropriate confidence bounds.
A concise synthesis of the defining clinical, manufacturing, and supply chain imperatives that stakeholders must prioritise to secure sustained access and value
Conclusion synthesising the most consequential insights about clinical positioning, supply chain imperatives, and strategic priorities for stakeholders
Vindesine Sulfate’s strategic relevance will be shaped by how well manufacturers and suppliers address three core dimensions: clinical fit, manufacturing and regulatory robustness, and supply chain resilience. Clinical teams will continue to weigh the API’s role against newer modalities, and thus evidence generation that clarifies comparative advantages will be pivotal. Manufacturing and quality capabilities are non-negotiable; organisations that invest in modernised processes and demonstrable compliance will be preferred partners for large health systems and research institutions.Supply chain strategies must account for geopolitical and trade-policy shifts, and proactive diversification of sourcing and regional manufacturing options will reduce exposure to tariff and transport volatility. Finally, successful commercial strategies will integrate segmentation-specific go-to-market plans, aligning dosage forms and distribution models with the operational needs of hospitals, clinics, and research centres. By prioritizing these dimensions, stakeholders can protect clinical supply, preserve access, and sustain competitive positioning in an increasingly demanding environment.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
19. China Vindesine Sulfate API Market
Companies Mentioned
- Accord Healthcare Ltd.
- Actavis Pharma, Inc.
- Alembic Pharmaceuticals Limited
- Amneal Pharmaceuticals, Inc.
- Apotex Inc.
- Aurobindo Pharma Limited
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- Eli Lilly and Company
- Fresenius Kabi AG
- Glenmark Pharmaceuticals Ltd.
- Hetero Drugs Limited
- Hikma Pharmaceuticals PLC
- Lannett Company, Inc.
- Lupin Limited
- Mylan N.V.
- Pfizer Inc.
- Sandoz International GmbH
- Strides Pharma Science Limited
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
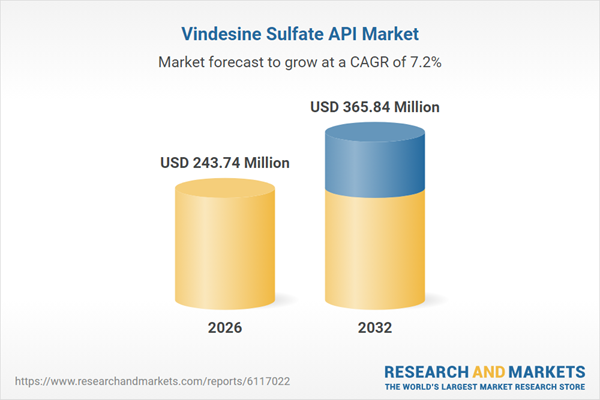
| Estimated Market Value ( USD | $ 243.74 Million |
| Forecasted Market Value ( USD | $ 365.84 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |