Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative orientation to autofocus endoscope technologies that clarifies clinical benefits, integration imperatives, and operational implications for healthcare stakeholders
Autofocus endoscope systems represent a pivotal convergence of optical engineering, digital imaging, and procedural ergonomics that aim to simplify visualization and improve clinical workflow across a broad range of specialties. By automating focal adjustments, these systems reduce manual intervention by clinicians, enabling more consistent image quality during dynamic procedures and reducing the cognitive burden associated with manual focusing. Consequently, autofocus technologies enhance procedural efficiency and contribute to improved documentation and training outcomes.Beyond the core clinical advantage, autofocus endoscopes interface with a broader technological stack that includes illumination systems, sensor arrays, processing algorithms, and user-interface design. These interfaces dictate both the intraoperative experience and post-procedural analytics capabilities, with implications for device lifecycle management, service models, and integration into hospital information systems. As hospitals and clinics increasingly prioritize standardized protocols and repeatable outcomes, autofocus functionality becomes a differentiator that supports operational reliability.
Given the interplay between clinical demand, regulatory oversight, and procurement priorities, stakeholders from clinical leaders to supply chain managers must evaluate autofocus endoscope adoption through a multidisciplinary lens. This report frames the technology in clinical, commercial, and logistical contexts, clarifying where autofocus capabilities deliver measurable value and where implementation hurdles require targeted mitigation strategies.
How rapid advances in automated imaging, illumination, and clinical practices are reshaping product design, procurement priorities, and competitive differentiation in endoscopy
The landscape for autofocus endoscopes is undergoing rapid transformation driven by a constellation of technological advances and evolving clinical imperatives. Automation of optical control has progressed alongside improvements in sensor sensitivity and computational imaging, enabling systems to maintain high-resolution focus across variable depths and during rapid instrument movement. At the same time, illumination technologies have shifted from legacy halogen sources toward more energy-efficient and color-stable alternatives, creating possibilities for smaller form factors and improved thermal profiles.Concurrently, clinical practice patterns are reshaping device requirements. Minimally invasive procedures continue to expand across specialties, increasing demand for flexible instruments that negotiate tortuous anatomy while preserving image fidelity. Infection prevention concerns and operational costs are accelerating interest in single-use alternatives, which in turn influence design trade-offs between disposability and optical performance. Interoperability with digital platforms-ranging from operating room video systems to machine-learning assisted interpretation-has emerged as a decisive factor for procurement committees seeking investments that will remain relevant to evolving digital workflows.
Finally, supply chain and manufacturing trends are prompting a rethink of sourcing strategies. Component miniaturization places greater emphasis on precision optics, custom sensor modules, and specialized illumination engines, while regulatory scrutiny and reimbursement considerations are shaping how quickly novel autofocus features are adopted into routine clinical practice. Together, these shifts are redefining competitive differentiation from purely optical performance to include service models, integration capabilities, and total cost of ownership considerations.
An integrated analysis of how evolving tariff policies are altering sourcing, pricing dynamics, and strategic manufacturing decisions across the autofocus endoscope supply chain
The cumulative impact of recent tariff actions and trade policy shifts has introduced new layers of complexity for manufacturers, distributors, and healthcare providers that depend on globally sourced components for autofocus endoscopes. Increased duties on certain imported components elevate input costs and create incentives to revisit sourcing footprints. In turn, procurement teams face pressure to balance cost containment with clinical requirements and the need for dependable supply continuity. Because many critical subassemblies-such as optical lenses, sensor modules, and high-intensity light sources-are concentrated in specific manufacturing clusters, tariff-related cost increases can ripple unevenly across the value chain.As a consequence, device OEMs are evaluating alternative responses that include nearshoring of assembly, qualification of secondary suppliers, and renegotiation of contractual terms with distributors and health systems. These responses often lengthen lead times for product introductions and may compel temporary decisions to prioritize higher-margin product lines or regions with more favorable trade terms. For healthcare organizations, the result is a need for more sophisticated procurement strategies that anticipate incremental cost pressures and incorporate contingency planning for clinical services that rely on affected product lines.
Moreover, tariff dynamics can accelerate broader strategic shifts, such as investment in single-use technologies to reduce the need for complex sterilization logistics that depend on imported reprocessing equipment, or partnerships to localize component production. Regulatory alignment and tariff classifications also affect time-to-market for products that must clear device regulations, adding another layer of planning for organizations seeking to mitigate the operational effects of trade policy changes.
Deep segmentation insight revealing how clinical specialty needs, device architectures, end-user priorities, illumination choices, and pricing tiers define adoption pathways and design trade-offs
Segmenting the autofocus endoscope landscape by clinical application, product architecture, end-user setting, illumination technology, and price positioning reveals distinct adoption patterns and design priorities. In applications such as Ear Nose and Throat procedures, which encompass bronchoscopy and laryngoscopy, device requirements emphasize maneuverability and fine optics to visualize narrow passageways, while gastrointestinal interventions distinguish between lower GI endoscopy and upper GI endoscopy, each with unique diameter constraints and durability expectations. Minimally invasive surgery applications, covering hysteroscopy, laparoscopy, and thoracoscopy, prioritize compatibility with operative tools and sustained image clarity during complex instrument interactions. Orthopedic procedures such as arthroscopy demand rigid optics with robust illumination, and urology workflows, including cystoscopy and ureteroscopy, balance small-diameter optics with irrigation management and durable materials to withstand repeated sterilization cycles.Product type segmentation further delineates market behavior. Flexible endoscopes, which vary by working diameter categories, are favored where navigation through tortuous anatomy is paramount, and device designers optimize distal optics, articulation, and channel compatibility accordingly. Rigid endoscopes are subdivided into angled and straight configurations, each serving specialized surgical tasks that require stable imaging and instrument alignment. Single-use products disrupt traditional lifecycle models by trading durability for disposability, often appealing to stakeholders seeking to reduce reprocessing risk and turn-around times.
End-user segmentation highlights divergent purchasing rationales. Ambulatory surgical centers generally emphasize throughput and cost efficiency, clinics favor compact, easy-to-use systems that enable outpatient diagnostics, hospitals prioritize system robustness, integration, and service contracts, and research institutes value extensibility for advanced imaging and experimental workflows. Illumination technology choices-ranging from halogen to LED to xenon-shape device thermal management, color rendering, and lifecycle costs, while price range considerations influence adoption curves, with premium purchases often justified by demonstrable workflow gains and long-term service economics.
A nuanced regional overview that links clinical adoption patterns, procurement behavior, and manufacturing realities across the Americas, Europe Middle East and Africa, and Asia Pacific markets
Regional dynamics play a decisive role in both demand formation and supply-side strategies for autofocus endoscopes. In the Americas, clinical adoption is influenced by high procedural volumes, advanced hospital infrastructure, and a procurement environment that increasingly prizes clinical evidence, service reliability, and total cost of ownership. This region often leads on adoption of single-use solutions and rapid integration of digital OR systems, reflecting both infection control priorities and investment capacities within health systems.Across Europe, the Middle East and Africa, heterogeneity defines market behavior. Western European markets emphasize regulatory compliance, clinical outcomes, and centralized procurement processes that reward demonstrable value and long-term service partnerships. Meanwhile, several markets within the Middle East show strong demand for turnkey surgical technology suites, and parts of Africa present both challenges and opportunities linked to infrastructure variability and the need for ruggedized equipment and scalable service models. These contrasts encourage manufacturers to pursue differentiated strategies by country and sub-region.
The Asia-Pacific region is characterized by fast-changing demand dynamics driven by rising surgical volumes, expanding healthcare infrastructure, and a strong emphasis on local manufacturing capabilities. Countries in North Asia continue to push technological advances in optics and imaging, while emerging markets prioritize cost-competitive solutions and scalable service networks. Supply chain considerations are particularly salient in this region, where proximity to component manufacturers can create advantages in lead time and customization, but where regulatory diversity requires careful market-entry planning.
A strategic assessment of how incumbent optical specialists and agile digital challengers compete through service models, integration strategies, and clinical validation to shape market positioning
Competitive dynamics in autofocus endoscopy are shaped by how companies balance core optical expertise with digital innovation and scalable service offerings. Established manufacturers that have long-standing relationships with hospitals often compete on system reliability, integrated service networks, and the breadth of compatible instruments. These incumbents increasingly invest in software-enabled features, clinical training programs, and bundled service contracts to protect installed bases and to create recurring revenue streams.At the same time, new entrants and specialized imaging firms are challenging traditional models by offering modular, software-centric solutions that emphasize ease of use, cloud-enabled analytics, and artificial intelligence-assisted visualization. These challengers often pursue partnerships with clinical centers of excellence to validate novel autofocus algorithms and to accelerate clinician adoption. Distribution strategies differ as well; some players focus on direct sales to large health systems, while others build channel partnerships to reach ambulatory centers and clinics.
Service and sterilization models remain a key competitive battleground. Organizations that can demonstrate lower lifecycle costs through efficient reprocessing workflows or that provide compelling single-use value propositions can gain traction in procurement cycles. Equally important is the ability to offer training, rapid technical support, and upgrade pathways that protect customer investments while enabling incremental adoption of advanced autofocus capabilities.
Actionable strategic priorities for manufacturers and health systems to accelerate clinical adoption, secure supply continuity, and capture long-term value from autofocus endoscope technologies
Industry leaders should pursue a multifaceted strategy to capture the value inherent in autofocus endoscope technologies while mitigating near-term operational risks. First, prioritize clinical evidence generation through targeted, specialty-specific studies that demonstrate workflow improvements, reductions in procedure time, or enhancements in diagnostic yield. Such evidence strengthens procurement narratives and smooths adoption across hospital committees. Second, diversify supply chains by qualifying alternate suppliers and evaluating nearshoring opportunities for critical components to reduce exposure to tariff-driven cost volatility.Third, design product portfolios for interoperability by ensuring seamless integration with prevailing operating room video systems and hospital IT infrastructures, and by offering flexible service models that range from capital purchases to subscription-based access. Fourth, develop a clear go-to-market segmentation strategy that aligns product form factors with end-user needs; for instance, invest in small-diameter flexible optics for GI and ENT workflows while maintaining rugged rigid options for orthopedics and certain surgical disciplines. Fifth, accelerate training and clinical support programs that reduce the learning curve for autofocus features and that embed value early in the customer relationship.
Finally, align commercial strategies with sustainability and sterilization considerations by offering evidence-based analyses of single-use versus reprocessable options and by exploring circular-economy solutions that mitigate environmental concerns while preserving clinical efficacy. Taken together, these measures position companies to respond nimbly to tariff impacts, regulatory shifts, and changing clinical expectations.
A comprehensive mixed-methods research approach combining clinician interviews, technical assessments, supply chain mapping, and policy analysis to generate actionable insights and validated recommendations
This research synthesizes insights from a blended methodology that integrates primary stakeholder engagements, technical device assessments, and secondary analysis of industry literature and policy developments. Primary research involved structured interviews with clinicians across specialties that commonly deploy endoscopes, procurement leaders within hospital systems and ambulatory centers, and technical experts responsible for device maintenance and sterilization. These engagements provided clarity on clinical workflows, procurement criteria, and pain points related to focus management and device ergonomics.Complementing primary input, technical assessments evaluated optical system architectures, illumination options, and user-interface designs to understand trade-offs between image performance and device form factors. Supply chain mapping traced component sourcing footprints and manufacturing dependencies to illuminate vulnerability points and potential mitigation pathways. Legal and policy reviews informed the assessment of tariff impacts and regulatory considerations that affect time-to-market and procurement decisions.
Finally, the research applied cross-sectional synthesis to reconcile divergent stakeholder perspectives and to produce actionable recommendations. Scenario planning exercises examined alternative responses to trade policy shifts and technology adoption curves, while validation panels with clinical and procurement leaders refined findings to ensure practical relevance and readiness for implementation.
A conclusive synthesis highlighting the clinical promise of autofocus systems and the strategic imperatives required to transform technical innovation into durable healthcare value
Autofocus endoscope technologies present a compelling opportunity to improve procedural consistency, reduce clinician workload, and enable richer documentation across a variety of clinical settings. Adoption decisions, however, hinge on more than optical performance alone; they require alignment with clinical evidence standards, sterilization and reprocessing frameworks, procurement priorities, and regional regulatory nuances. Stakeholders who integrate these perspectives into product development and market-entry strategies will be better positioned to realize the clinical and commercial benefits of autofocus capabilities.Supply chain volatility and trade policy developments underscore the need for resilient sourcing strategies and flexible commercial models that can absorb near-term cost shocks while preserving access to critical technologies. In parallel, companies that emphasize interoperability, robust service offerings, and targeted clinical validation will gain competitive advantage. Ultimately, translating autofocus innovations into sustained clinical value depends on coordinated investments in evidence generation, clinician training, and operational integration to ensure that advanced imaging capabilities translate into measurable improvements in patient care and system efficiency.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Autofocus Endoscope Market
Companies Mentioned
- Ambu A/S
- Arthrex, Inc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Clarus Medical LLC
- CONMED Corporation
- Cook Medical LLC
- ELMED Medical Systems
- EndoMed Systems GmbH
- Ethicon, Inc.
- Fujifilm Holdings Corporation
- Intuitive Surgical, Inc.
- Johnson & Johnson Services, Inc.
- KARL STORZ SE & Co. KG
- Medtronic plc
- Mindray Medical International Limited
- NeoScope Inc.
- Olympus Corporation
- Pentax Medical Company
- Pristine Surgical LLC
- Richard Wolf GmbH
- Smith & Nephew plc
- SonoScape Medical Corp.
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
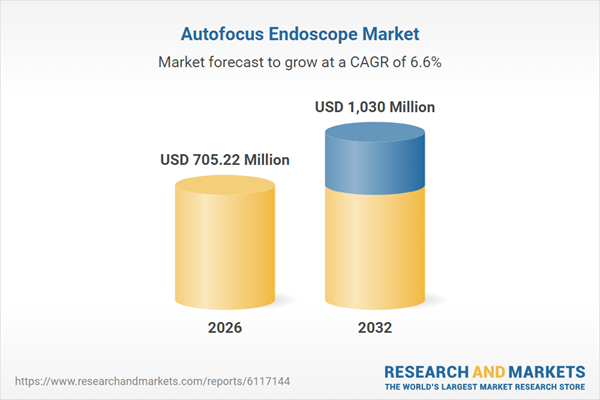
| Estimated Market Value ( USD | $ 705.22 Million |
| Forecasted Market Value ( USD | $ 1030 Million |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |