Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to clinical rationale, therapeutic role, and contemporary relevance of αIIbβ3 antagonists amid evolving antithrombotic practice patterns
The αIIbβ3 antagonists class occupies a pivotal role in thrombosis management and interventional cardiology, with long-standing clinical utility in high-acuity settings. These agents target the platelet integrin αIIbβ3 to inhibit fibrinogen-mediated platelet aggregation, serving as critical therapeutics during acute coronary syndromes and percutaneous coronary interventions where rapid platelet inhibition mitigates ischemic complications. Historically, intravenous monoclonal antibodies and peptide- and small-molecule antagonists have been integral to peri-procedural antiplatelet strategies, supporting improved procedural outcomes when used judiciously alongside dual antiplatelet regimens.In recent years the landscape has evolved as clinicians and developers place increasing emphasis on safety profiles, delivery formats, and integration with evolving antithrombotic standards of care. Emerging evidence and regulatory activity have prompted a reassessment of where αIIbβ3 antagonists add incremental clinical value versus newer P2Y12 inhibitors and antithrombotic strategies. Consequently, stakeholders across clinical, regulatory, and commercial functions are reexamining development priorities for next-generation αIIbβ3-directed therapies and the potential for differentiated formulations that reconcile efficacy with bleeding risk and logistical constraints of acute care settings.
Analysis of clinical, technological, and care-delivery forces reshaping strategic priorities and development pathways for αIIbβ3 antagonist therapies
The therapeutic and commercial landscape for αIIbβ3 antagonists is experiencing transformative shifts driven by clinical priorities, technological advances, and changing patterns of care delivery. First, there is a clear clinical pivot toward minimizing bleeding risk while preserving antithrombotic efficacy, which has pressured developers to re-evaluate dosing paradigms, infusion profiles, and agent selectivity. As a result, development programs increasingly favor agents or delivery systems that offer rapid on/off pharmacodynamics or targeted local delivery to reduce systemic exposure.Second, the maturation of alternative antiplatelet classes and the expansion of potent oral agents have altered the contexts in which intravenous αIIbβ3 antagonists are used, concentrating demand in high-acuity settings such as complex percutaneous coronary interventions and select acute coronary syndromes. Third, manufacturing innovation and biologics optimization are enabling more consistent supply and the potential for biosimilar monoclonal antibodies, which in turn are reshaping commercial access models. Finally, health system priorities-namely value-based procurement, shorter procedural stays, and a growing emphasis on ambulatory care-are catalyzing interest in administration routes and dosage forms that align with constrained inpatient resources. Taken together, these shifts are redefining therapeutic positioning, investment focus, and partnership models across the ecosystem.
Assessment of how evolving United States tariff policies are reshaping supply chain resilience, procurement strategies, and commercial planning for αIIbβ3 antagonist stakeholders
Recent tariff adjustments and trade policy developments in the United States have introduced new cost and operational variables for manufacturers, distributors, and healthcare organizations that rely on internationally sourced active pharmaceutical ingredients and finished-dose components. These tariff dynamics have amplified supply-chain scrutiny, leading many organizations to reassess suppliers, qualify alternate sources, and in some cases accelerate regional manufacturing initiatives to mitigate exposure to import-related volatility. Consequently, procurement timelines have lengthened as quality and compliance checks accompany supplier diversification efforts, and contracting teams have had to allocate additional bandwidth to tariff classification and landed-cost modeling.Clinically, increased input costs and logistical hurdles can influence formulary negotiations and hospital purchasing behaviors, prompting closer evaluation of cost-offsets such as reduced complication rates or shorter procedural times. At the same time, developers face rising production expenses that can constrain pricing flexibility for new launches unless offset by efficiency gains or strategic partnerships. In the regulatory domain, companies are prioritizing resilient supply chains and documentation readiness to ensure continuity of supply under changing tariff regimes. In sum, cumulative tariff-related pressures are altering commercial calculus and operational planning across the value chain, encouraging greater vertical integration, regional capacity investments, and collaborative procurement approaches to preserve access to critical αIIbβ3 therapies.
Integrated segmentation analysis revealing how application, product type, administration route, end-user, distribution, dosage form, and patient demographics shape strategic priorities
A nuanced view of segmentation reveals how clinical application, product type, administration route, end-user setting, distribution pathways, dosage form, and patient demographics collectively influence development priorities and commercialization strategies. Based on application, therapeutic use cases include Acute Coronary Syndrome, Percutaneous Coronary Intervention, and Thrombotic Disorders, with Thrombotic Disorders further encompassing Deep Vein Thrombosis, Pulmonary Embolism, and Stroke Prophylaxis; this distribution underscores where clinicians prioritize rapid, reliable platelet inhibition and where opportunities exist for differentiated risk-benefit profiles. Based on product type, the landscape comprises Monoclonal Antibodies, Peptide Based Antagonists, and Small Molecule Antagonists, with Monoclonal Antibodies further represented by agents such as abciximab, Peptide Based Antagonists further exemplified by eptifibatide, and Small Molecule Antagonists further typified by tirofiban; each product subclass carries distinct manufacturing, regulatory, and clinical-use implications that inform strategic choices.Based on route of administration, differentiated demand for Intravenous versus Oral formats dictates development emphasis on rapid-onset agents for procedural settings versus sustained therapies for outpatient management. Based on end user, adoption patterns vary markedly across Ambulatory Surgical Centers, Clinics, Hospitals, and Research Institutions as each setting imposes unique operational constraints and procurement practices. Based on distribution channel, accessibility is shaped by availability through Hospital Pharmacies, Online Pharmacies, and Retail Pharmacies, which affects hospital formulary dynamics and outpatient continuity. Based on dosage form, Injection and Oral Tablet options create distinct patient-experience and logistical considerations that impact adherence and administration workflows. Based on patient type, stratification across Adult, Geriatric, and Pediatric cohorts requires tailored clinical evidence and dosing strategies to address differential risk profiles and regulatory expectations. An integrated segmentation perspective therefore informs where clinical differentiation and commercial interventions are most likely to succeed.
Regional perspective on adoption drivers, regulatory environments, and commercialization approaches across the Americas, Europe Middle East & Africa, and Asia-Pacific markets
Regional dynamics play a central role in clinical adoption patterns, regulatory pathways, and commercial strategies for αIIbβ3 antagonists. In the Americas, the care pathway emphasis on interventional cardiology and well-established hospital networks drives sustained demand for peri-procedural agents, while regulatory frameworks and reimbursement mechanisms incentivize evidence that demonstrates improved procedural outcomes and reduced complications. In Europe, Middle East & Africa, heterogeneous regulatory environments and variable access to advanced interventional services create differentiated adoption curves; in some markets structured national procurement and tendering processes favor cost-effective formulations and biosimilar entrants, whereas others emphasize rapid access to innovative biologics.In the Asia-Pacific region, expanding cardiovascular disease burden, growing interventional capacity, and investments in domestic pharmaceutical manufacturing present both high-volume opportunity and competitive manufacturing advantages. Across these regions, divergent payer structures, hospitalization practices, and supply chain footprints necessitate region-specific commercialization blueprints that account for regulatory requirements, clinician training needs, and local procurement practices. Consequently, developers must align clinical development, evidence generation, and market access strategies with the distinct drivers present in each region to maximize adoption and ensure continuity of patient care.
Competitive dynamics and strategic behaviors among biologics developers, specialty injectables players, and innovators shaping clinical differentiation and market access strategies
Competitive dynamics across the αIIbβ3 antagonist space are shaped by distinct categories of players: established biologic developers, specialty injectables manufacturers, small-molecule innovators, and clinical-stage biotech enterprises pursuing differentiated mechanisms or delivery formats. Established developers often leverage clinical legacy and existing hospital relationships to maintain presence in peri-procedural settings, while specialty manufacturers focus on manufacturing efficiency and tender-based distribution to reach hospital formularies. Innovators prioritize translational programs that improve therapeutic windows, explore targeted delivery modalities to reduce systemic bleeding risk, and investigate oral or subcutaneous alternatives designed for outpatient use. Additionally, cross-sector partnerships between pharmaceutical firms and device makers are emerging to optimize combination strategies for interventional cardiology.Investor and corporate strategies increasingly reflect a two-pronged approach: defend core monoclonal antibody franchises through lifecycle management and efficiency gains, while incubating next-generation assets that address unmet needs in safety, convenience, and cost-effectiveness. Licensing, co-development, and regional manufacturing alliances are common mechanisms to accelerate market entry and share development risk. At the same time, a growing emphasis on real-world evidence and health-economic data drives post-approval investment to support reimbursement and formulary inclusion across disparate health systems.
Action-oriented strategic framework for industry leaders to fortify supply resilience, prioritize safety-driven innovation, and accelerate market access for αIIbβ3 agents
Industry leaders should adopt a multi-dimensional playbook that balances near-term operational resilience with medium-term innovation to sustain clinical relevance and commercial viability. First, diversify and qualify multiple suppliers for APIs and critical components while investing in regional manufacturing capacity to buffer tariff and trade-related volatility; this reduces single-source risk and shortens supply lead times. Second, prioritize clinical programs that demonstrate improved safety profiles or enable outpatient administration, since these attributes align with health system imperatives to lower procedural burden and shorten hospital stays.Third, pursue strategic partnerships to share development risk and accelerate access in priority markets, leveraging regional collaborators for regulatory navigation and channel penetration. Fourth, invest in robust real-world evidence generation and health-economic modeling to articulate value to payers and procurement bodies, thereby smoothing formulary negotiations. Fifth, enhance product differentiation through formulation innovation, such as rapid-onset delivery systems or administration modalities that simplify use in ambulatory settings. Finally, align commercial models with changing procurement behaviors by offering value-based contracting options and flexible supply agreements that reflect institutional priorities and budget cycles. Implementing these strategic moves in a coordinated manner will strengthen resilience and position organizations to capture opportunity as clinical and delivery paradigms evolve.
Robust mixed-methods research approach combining clinician interviews, regulatory dossier review, clinical literature synthesis, and supply-chain analysis to underpin findings
This study synthesizes primary and secondary intelligence to construct an actionable evidence base that supports strategic planning and decision-making. Primary research activities include structured interviews with clinicians, hospital pharmacists, regulatory specialists, and industry executives to capture frontline perspectives on clinical utility, procurement dynamics, and unmet needs. Secondary research incorporates regulatory filings, peer-reviewed clinical literature, clinical trial registries, and publicly available drug labeling to validate mechanism-specific attributes and safety considerations. In addition, patent literature and manufacturing filings were reviewed to assess production capabilities and freedom-to-operate considerations.Analytical techniques included qualitative triangulation to reconcile diverse stakeholder views and thematic synthesis to identify emergent trends in clinical practice and product development. Supply-chain analyses examined sourcing geographies, packaging and cold-chain requirements, and distribution pathways to elucidate operational vulnerabilities. Quality-control processes included cross-validation of interview findings with literature sources and archival regulatory documents, ensuring reproducibility and rigor. Together, these methods produced a comprehensive, defensible perspective designed to inform tactical and strategic decisions without relying on speculative market sizing or proprietary estimates.
Concise conclusion summarizing clinical imperatives, operational pressures, and strategic levers that will determine future leadership in the αIIbβ3 antagonist landscape
αIIbβ3 antagonists maintain an important clinical niche where rapid, potent platelet inhibition is essential, particularly in complex interventional cardiology and select high-risk thrombotic indications. The interplay between clinical need, safety imperatives, and operational realities is prompting a reorientation of development priorities toward agents and formats that can deliver efficacy while minimizing bleeding and logistical burdens. Supply-chain disruptions and evolving trade dynamics further underscore the importance of manufacturing agility and regional resilience as determinants of commercial success.Moving forward, stakeholders who integrate targeted clinical development, manufacturing diversification, and compelling real-world evidence will be best positioned to navigate the shifting landscape. By aligning product attributes with the procedural contexts and care pathways where αIIbβ3 antagonists deliver unique value, organizations can preserve clinical relevance and create differentiated offerings that address both clinician requirements and payer expectations. Ultimately, strategic clarity and operational adaptability will determine who captures long-term opportunity as treatment paradigms and procurement practices continue to evolve.
Table of Contents
20. ResearchStatistics
21. ResearchContacts
22. ResearchArticles
23. Appendix
Companies Mentioned
- Alexion Pharmaceuticals, Inc.
- Biogen Inc.
- CeleCor Therapeutics, Inc.
- Centocor, Inc.
- COR Therapeutics, Inc.
- Correvio Pharma Corp.
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- GlaxoSmithKline plc
- Indalo Therapeutics, Inc.
- Janssen Biotech, Inc.
- Medicure Inc.
- Merck & Co., Inc.
- Merck KGaA
- Millennium Pharmaceuticals, Inc.
- Pliant Therapeutics, Inc.
- Portola Pharmaceuticals, Inc.
- Schering-Plough Corporation
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
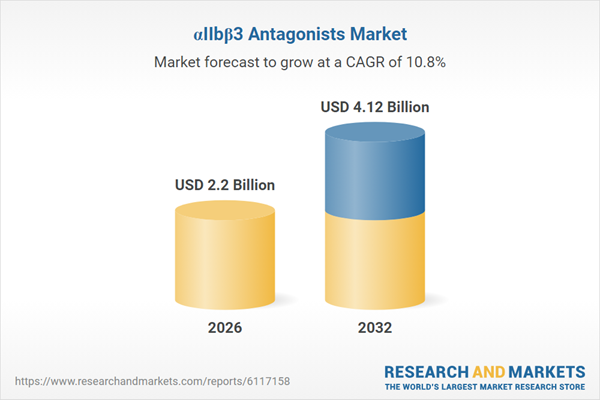
| Estimated Market Value ( USD | $ 2.2 Billion |
| Forecasted Market Value ( USD | $ 4.12 Billion |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |