Speak directly to the analyst to clarify any post sales queries you may have.
A focused orientation to why sterile glass vials are now strategic engineered components that influence product stability, regulatory compliance, and supply chain resilience
An efficient introduction to the sterile glass vial landscape begins by framing the product’s critical role in modern healthcare and laboratory ecosystems. Sterile glass vials serve as primary containment for a wide range of biologics, small-molecule injectables, and diagnostic reagents, and their material properties directly affect pharmaceutical stability, sterility assurance, and downstream handling. As therapeutic modalities diversify, demands placed on vial performance have shifted: enhanced chemical resistance, stricter particulate control, and compatibility with cold-chain logistics are now baseline expectations rather than optional differentiators.Regulatory authorities have tightened expectations around container integrity, extractables and leachables profiling, and manufacturing traceability, which has elevated the importance of validated production processes and supply chain transparency. Simultaneously, end-user preferences-spanning clinics, hospitals, and labs-have evolved toward packaging that simplifies preparation and reduces handling risk. These convergent forces have reshaped procurement criteria and supplier selection frameworks.
Taken together, these developments underscore why packaging teams, quality leaders, and procurement executives must regard sterile glass vials not solely as passive containers but as engineered components critical to therapeutic efficacy and patient safety. This perspective informs subsequent analysis of technological shifts, trade policy impacts, segmentation dynamics, regional considerations, corporate strategies, and actionable recommendations presented in the remainder of this summary.
Material innovations, regulatory tightening, and supply chain reconfiguration are converging to transform sterile glass vial production, qualification, and commercial partnerships
The landscape for sterile glass vials is being reshaped by several transformative shifts that operate across materials science, regulatory enforcement, and supply chain architecture. Advances in glass composition and surface treatments have driven improved resistance to breakage and interaction with biologics, and these material innovations are being paired with precision manufacturing technologies to reduce particulate generation and tighten dimensional tolerances. At the same time, packaging is adapting to the proliferation of sensitive modalities such as therapeutic proteins and next-generation vaccines, which impose stricter requirements for extractables, leachables, and cold-chain compatibility.Regulatory frameworks are converging around stronger documentation requirements, batch traceability, and risk-based approaches to packaging qualification. This regulatory tightening incentivizes investments in validated processes and digital record-keeping to demonstrate compliance. Concurrently, commercial pressures and geopolitical dynamics have placed a premium on supply chain resilience, encouraging buyers to diversify sources and to seek suppliers with local or regional manufacturing footprints.
Operationally, the industry is experiencing a shift toward collaborative product development between vial manufacturers and pharmaceutical developers, enabling co-validated solutions that accelerate clinical and commercial timelines. Taken together, material innovation, regulatory stringency, supply chain reconfiguration, and collaborative development models define the current transformative environment and set the context for strategic decision-making.
Evolving tariff dynamics are prompting strategic shifts toward supplier diversification, local capacity decisions, and contract structures that protect supply continuity and cost stability
Recent tariff and trade measures have introduced new operational considerations for organizations that source sterile glass vials across borders. Changes in import duties affect landed costs, supplier competitiveness, and the relative attractiveness of local manufacturing versus international procurement. In response to tariff pressures, many buyers have pursued strategies that emphasize supplier diversification, nearshoring, and long-term contracting to stabilize input costs and maintain predictable access to critical packaging components.Proactively, manufacturers have reassessed their production footprints, evaluating options to relocate or expand capacity in regions with favorable trade relationships and logistics advantages. These strategic shifts often involve balancing higher local production costs against reduced exposure to tariff volatility and enhanced supply continuity. At the same time, procurement teams have adapted by tightening inventory policies for critical SKUs and by implementing dual-sourcing arrangements that mitigate single-source risk.
Beyond cost implications, tariffs influence negotiation dynamics and supplier consolidation decisions. Buyers and suppliers are increasingly structuring agreements that incorporate flexible pricing mechanisms, defined escalation clauses tied to raw material indices, and shared-risk provisions to manage the commercial impact of trade policy changes. As such, trade measures represent both a challenge and a catalyst for more resilient sourcing strategies and commercially sophisticated supply agreements.
Detailed segmentation highlighting differentiated technical requirements and procurement behaviors across product types, applications, end uses, closure designs, and distribution pathways
Segmentation analysis reveals differentiated requirements and value drivers that suppliers and buyers must address across product, application, end use, closure type, and distribution channels. When examined by product type-including ampoule vials, Type I borosilicate vials, Type II soda lime vials, and Type III tubed vials-performance expectations vary from ultra-high chemical resistance to cost-optimized containment, which in turn informs material selection and process controls. Application segmentation highlights distinct specification profiles; biotechnology, cosmetic, food and beverage, laboratory, and pharmaceutical uses impose unique demands, and within pharmaceuticals a further focus on therapeutic proteins and vaccines elevates concerns around cold-chain integrity, extractables, and sterility assurance.End use channels such as clinics, contract research organizations, diagnostic laboratories, and hospitals introduce operational criteria that affect preferred vial formats, closure compatibility, and packaging ergonomics. Decisions about closure type-whether aluminum seal, flip off cap, melamine closure, or rubber stopper-are driven by the need to balance sterile integrity, ease of access, and compatibility with automated dosing systems. Distribution channel dynamics also influence buying behavior: direct purchase arrangements tend to favor long-term partnerships and bespoke specifications, distributors provide breadth and inventory coverage for diverse customers, and online channels offer convenience and agility for lower-volume or specialized procurement.
Understanding how these segmentation dimensions interact allows suppliers to prioritize investments in specific product families, closure R&D, and distribution strategies while enabling buyers to align procurement frameworks with clinical and operational priorities. Ultimately, segmentation-informed strategies reduce technical risk and improve time-to-clinic for regulated therapies.
Regional dynamics from the Americas through Europe, Middle East & Africa to Asia-Pacific shape supplier selection, qualification timelines, and production footprints
Regional dynamics shape priorities across innovation, regulatory compliance, and supply chain strategy. In the Americas, demand drivers include a concentration of biopharmaceutical development and an emphasis on rapid clinical translation, which supports demand for high-specification borosilicate vials and advanced closure systems. This environment encourages close collaboration between vial suppliers and drug developers to co-validate packaging solutions and to support accelerated clinical timelines.In Europe, Middle East & Africa, regulatory harmonization efforts and stringent import controls influence both supplier selection and qualification timelines. Manufacturers operating in this region often emphasize documented quality systems, regional manufacturing footprints, and logistical reliability to meet multi-jurisdictional requirements. The region also presents a diverse demand profile that ranges from high-volume pharmaceutical production to specialized diagnostic and laboratory applications.
Asia-Pacific presents a mix of established industrial capacity and rapid growth in biomanufacturing, with several markets investing in local packaging capabilities to support domestic clinical pipelines and to serve export demand. In this region, cost competitiveness remains a key consideration, but it is increasingly balanced with investments in quality assurance and process validation as local producers aim to serve global customers. Across all regions, proximity to biopharma clusters, the availability of qualified materials, and logistical connectivity are central determinants of supplier attractiveness and sourcing strategy.
Supplier strategies are converging around technical capability, validated processes, regional capacity, and partnership models that accelerate client development and reduce supply risk
Corporate positioning and capability sets among leading suppliers reflect a combination of technical horsepower, capacity scale, and service-oriented offerings. Key players are investing in advanced material science, high-precision forming processes, and enhanced quality management systems to meet stricter regulatory expectations and to support sensitive therapeutic modalities. These investments include upgraded cleanroom environments, automated inspection technologies, and enhanced traceability systems that enable batch-level provenance and rapid recall readiness when necessary.Strategic differentiation is also emerging through service models. Some companies emphasize co-development and technical support, partnering with pharmaceutical clients early in formulation and filling development to optimize vial-closure compatibility and to de-risk qualification. Others prioritize flexible manufacturing and regional footprint expansion to offer low lead times and mitigated trade exposure. Mergers and strategic alliances are being used selectively to access complementary capabilities, while select niche specialists focus on high-value segments such as vaccine-specific vial formats or ultra-low-particulate production lines.
For buyers, the combination of technical competence, supply reliability, and collaborative service capabilities has become the primary criteria for supplier selection. Suppliers that can demonstrate validated processes, responsive technical support, and regional logistics flexibility are increasingly preferred partners for regulated product programs and rapid development timelines.
Practical strategic actions for leaders to strengthen quality systems, diversify supply, integrate packaging early in development, and structure resilient commercial agreements
Industry leaders should adopt a proactive set of strategic actions to navigate technical, regulatory, and commercial complexities while preserving agility. First, prioritize investments in validated manufacturing processes and digital traceability systems that document provenance from raw material to finished vial. This reduces regulatory friction and strengthens commercial positioning by enabling transparent evidence of quality. Second, adopt a supplier diversification strategy that blends local capacity with global partners; nearshoring selected SKUs can reduce exposure to trade volatility and compress lead times while international partnerships maintain cost flexibility and scale.Third, integrate packaging selection early in drug development programs by embedding cross-functional teams that include formulation scientists, quality assurance, and supply chain professionals. This early integration accelerates packaging qualification and avoids late-stage changes that are costly and time-consuming. Fourth, explore collaborative development agreements with specialized vial makers to co-validate materials and surface treatments for sensitive biologics, thereby reducing technical risk and shortening validation timelines. Fifth, optimize commercial contracts by including flexible pricing mechanisms, service level agreements, and contingency clauses tied to logistics disruptions, which create shared incentives and clearer expectations.
Finally, maintain a continuous improvement posture: invest in inspection automation, particulate control technologies, and closure compatibility testing, and periodically reassess closure and distribution strategies to align with evolving clinical workflows. These actions collectively improve resilience, reduce time-to-deployment, and protect product quality across the lifecycle.
A rigorous research framework that blends primary expert engagement, technical literature review, and methodical validation to produce decision-ready insights for packaging and procurement teams
The research approach combines qualitative and quantitative techniques with rigorous validation to ensure reliability and applicability. Primary inputs include structured interviews with packaging engineers, quality assurance leads, procurement heads, and regulatory specialists across pharmaceutical and biotech organizations. These conversations informed an understanding of specification drivers, supplier selection criteria, and real-world constraints in development and manufacturing environments. Secondary inputs included technical literature, regulatory guidance documents, and publicly available product literature to validate technical claims and to triangulate technological trends.Data synthesis involved cross-referencing technical specifications, material property data, and published regulatory expectations to identify commonalities and divergences across product types and applications. Validation steps included follow-up discussions with independent subject matter experts and cross-checks against documented quality frameworks and industry best practices. The methodology emphasizes transparency in source attribution, clear articulation of assumptions used in interpretation, and a recognition of the limits of publicly available data when assessing proprietary manufacturing processes.
Where gaps in direct observation existed, the analysis relied on expert consensus and documented case studies to infer plausible operational responses. The final deliverable integrates these multi-source inputs into a coherent set of insights and recommendations designed to be operationally relevant for decision-makers responsible for packaging selection, supplier qualification, and supply chain strategy.
A concise synthesis of why integrated packaging strategy, validated processes, and resilient sourcing turn sterile glass vials from a risk factor into a competitive advantage
In closing, sterile glass vials are no longer commoditized items; they are engineered components that materially influence product stability, regulatory compliance, and operational efficiency. The interplay between material innovation, regulatory expectations, and supply chain dynamics necessitates that manufacturers, developers, and procurement teams adopt an integrated approach to packaging selection and supplier engagement. By aligning technical specifications with end-use requirements and by proactively managing geographic sourcing strategies, organizations can reduce project risk and support accelerated product timelines.Key themes that emerge are the importance of validated processes and traceability, the value of early cross-functional packaging integration in development programs, and the need for contract structures that address trade and logistics volatility. Companies that invest in technical differentiation, maintain flexible sourcing, and foster collaborative supplier relationships will be best positioned to support evolving therapeutic modalities and complex distribution requirements.
Ultimately, strategic attention to vial materials, closure compatibility, and supply continuity converts packaging from a potential bottleneck into a competitive enabler for product safety, patient outcomes, and commercial success.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- Acme Vial & Glass Company, LLC
- Adelphi Healthcare Packaging
- Arab Pharmaceutical Glass Co.
- Ardagh Group S.A.
- Beatson Clark Ltd
- Bormioli Pharma S.p.A.
- Corning Incorporated
- DWK Life Sciences GmbH
- Gerresheimer AG
- Kinde Engineering Co., Ltd.
- Kishore Group
- Nippon Electric Glass Co., Ltd.
- Nipro Corporation
- Origin Pharma Packaging
- Otsuka Glass Co., Ltd.
- Owens-Illinois, Inc.
- Owens-Illinois, Inc.
- Pacific Vial Manufacturing, Inc.
- PGP Glass
- Piramal Enterprises Ltd
- Qorpak, Inc.
- SCHOTT AG
- SGD Pharma S.A.S.
- Shandong Pharmaceutical Glass Co., Ltd.
- Stevanato Group S.p.A.
- Stoelzle Oberglas GmbH
- West Pharmaceutical Services, Inc.
- Şişecam Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
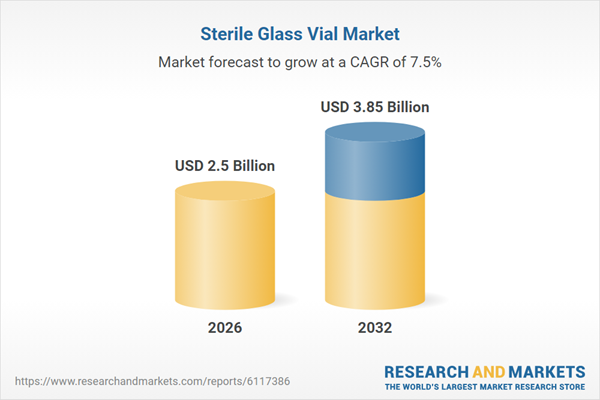
| Estimated Market Value ( USD | $ 2.5 Billion |
| Forecasted Market Value ( USD | $ 3.85 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |