Speak directly to the analyst to clarify any post sales queries you may have.
A concise strategic primer on ultrasonic oxygen flow metering that outlines its technical advantages, operational roles, and strategic relevance to modern process systems
Ultrasonic oxygen flow meters have matured from niche laboratory instruments into versatile field devices that address an expanding set of industrial and healthcare requirements. These meters exploit ultrasonic time-of-flight and Doppler principles to measure gas flow non-invasively, offering low maintenance, high reliability, and the ability to operate across varying pressure and temperature envelopes. As stakeholders demand more accurate gas analytics without intrusive interventions, ultrasonic technologies increasingly displace traditional mechanical and thermal flow measurement approaches.Adoption is driven by tighter regulatory environments, greater emphasis on safety and process optimization, and operational imperatives such as reduced downtime and lower total cost of ownership. Integration with digital control systems and networked telemetry has transformed many meters into nodes in distributed monitoring architectures, delivering continuous data streams for control rooms and remote diagnostics. Consequently, procurement decisions are as much about data fidelity, lifecycle support, and interoperability as they are about primary measurement performance.
This introduction frames the technology’s core value proposition: contactless measurement, resilience in challenging process conditions, and compatibility with modern automation ecosystems. For executives and technical leads, the critical takeaway is that ultrasonic oxygen flow metering is no longer a specialist option; it is a strategic instrument for improving safety, regulatory compliance, and process efficiency across multiple sectors.
How sensor miniaturization, edge analytics, and evolving regulatory expectations are reshaping procurement priorities and competitive dynamics in flow metering
The past several years have seen transformative shifts across supply chains, technology architectures, and regulatory expectations that directly affect the ultrasonic oxygen flow meter landscape. Advances in sensor microelectronics and low-power embedded processing have enabled higher sampling rates and on-board analytics, which in turn allow predictive maintenance and anomaly detection to be embedded at the device level. Simultaneously, the rise of industrial edge computing and standardized communications stacks has reduced integration friction for facility operators, making deployment cycles shorter and more predictable.On the commercial side, buyers increasingly evaluate instruments through the lens of lifecycle services and data governance rather than unit cost alone. This has encouraged vendors to bundle calibration-as-a-service, remote diagnostics, and firmware lifecycle management into their offerings. Regulatory evolution, particularly around emissions, workplace safety, and medical device oversight, has also shifted priorities toward meters that can demonstrate traceability, reproducibility, and secure data handling. These combined forces are accelerating consolidation of functional requirements and raising the bar for verification, validation, and post-sale support.
Market entrants that prioritize software-enabled value, open interoperability, and robust compliance pathways are positioned to capture incremental adoption. Looking ahead, the convergence of digitalization, stricter operational standards, and hardware miniaturization will continue to reshape procurement criteria and competitive dynamics across applications and channels.
Assessing how tariff shifts and trade policy changes are influencing supply chain resilience, sourcing strategies, and procurement risk for instrument manufacturers
Policy decisions in major trade jurisdictions, including tariff adjustments proposed or implemented through 2025, have introduced additional complexity across supply, procurement, and pricing for flow metering equipment and subcomponents. Tariffs can affect cost structures along the value chain by increasing the landed cost of imported sensors, custom electronics, and subassemblies, which forces original equipment manufacturers and distributors to reassess sourcing strategies. In response, many vendors have accelerated supplier diversification and sought closer vertical integration to control exposure to cross-border policy risk.The cumulative impact of tariff activity tends to manifest through three interlinked pressures. First, device manufacturers face higher input costs that may be passed downstream or absorbed depending on competitive dynamics and contract rigidities. Second, procurement teams shift to emphasize regional supply resilience and shorter lead times, which often elevates locally manufactured or assembled offerings in request-for-proposal evaluations. Third, service models evolve to emphasize retrofit and upgradeability, as customers prefer to extend the useful life of existing assets rather than procure higher-cost replacements.
Importantly, tariff effects are mitigated by strategic responses that do not rely solely on price adjustments. Design for modularity, increased use of standardized commodity components, and strategic inventory hedging reduce vulnerability to sudden trade policy shifts. For organizations planning capital investments or long-term supplier partnerships, the prudent course is to evaluate supplier footprints, contractual protections, and the ability to provide compliant documentation that supports alternative sourcing and local assembly when necessary.
Mapping product, application, technology, channel, and end-use segmentation to reveal differentiated product strategies and clear deployment trade-offs
Segmentation reveals where performance expectations and go-to-market strategies must diverge to capture value effectively. When evaluated by end use, devices deployed for environmental monitoring face different durability and certification needs compared with those used in fire safety, healthcare, or industrial processes. Environmental monitoring splits further into air quality and water quality uses, each with distinct detection thresholds, contamination risk profiles, and data reporting standards. Fire safety deployments emphasize rapid detection and rugged control-system integration, with the domain further differentiated between control-oriented systems and standalone detection instruments. Healthcare applications separate into homecare and hospital contexts where usability, patient safety features, and regulatory class differ substantially. Industrial end uses span automotive, chemical, and oil and gas, each imposing unique pressure, temperature, and hazardous-area requirements that determine materials and certification strategies.Product segmentation between fixed and portable units requires different design priorities and sales motions. Fixed meters, whether panel mounted or wall mounted, tend to be integrated into facility automation systems and are procured through project or capital-expenditure channels with extended service contracts. Portable meters, either handheld or on mobile trolleys, are sold through distributors and service organizations that prioritize ease of use, rapid calibration, and ruggedness for field diagnostics.
Application-focused segmentation-covering environmental monitoring, medical oxygen delivery, process control, and safety equipment-clarifies functional requirements and compliance pathways. Environmental monitoring divides into air and water monitoring, medical oxygen delivery separates homecare and hospital use where device usability and alarm management differ, process control distinguishes chemical from pharmaceutical processes with contrasting traceability and purity demands, and safety equipment spans fire suppression and leak detection where response times and integration with suppression systems are critical.
Technological segmentation clarifies installation and measurement trade-offs. Clamp-on technologies, whether portable clamp-on or ultrasonic clamp-on, offer non-intrusive installation and fast deployment, while inline options-full-bore and insertion type-provide higher baseline accuracy and are favored when long-term integration and minimal measurement variance are priorities. Finally, sales channel segmentation across direct, distributor, and online routes shapes margins and service expectations. Direct channels, including aftermarket and OEM relationships, facilitate deep technical collaborations and bespoke integration, whereas distributors-both local and national-provide breadth and responsiveness. Online channels via manufacturer websites or third-party platforms expand reach but require robust digital content, warranty clarity, and fulfillment capabilities.
Collectively, these segmentation lenses demonstrate that successful product strategies require aligning certification, installation modality, and post-sale service with the specific demands of the target end use and application environment.
Region-specific commercial, regulatory, and operational dynamics that shape procurement priorities and aftermarket service expectations across global markets
Regional dynamics materially influence adoption patterns, partner ecosystems, and regulatory expectations across the ultrasonic oxygen flow meter landscape. In the Americas, demand drivers are shaped by industrial modernization programs, a strong emissions compliance agenda, and an established ecosystem of instrumentation distributors and service providers. Buyers in this region frequently prioritize integration with existing automation systems and expect comprehensive lifecycle support and regional calibration capabilities.Europe, Middle East & Africa presents a heterogeneous environment where stringent European regulatory frameworks drive high levels of compliance and traceability for hospital and environmental applications, while emerging markets within the region emphasize cost-effectiveness and ruggedness. The Middle East’s energy sector places a premium on instruments capable of operating in extreme conditions, and Africa’s growing industrial base often values modular, serviceable solutions that can be maintained with limited local specialist support.
Asia-Pacific combines rapid industrial expansion with aggressive adoption of digital monitoring, particularly in manufacturing hubs and urban environments concerned with air quality. The region’s supplier density and manufacturing capabilities also make it a focal point for component sourcing, assembly, and innovation. Across all regions, the balance between local manufacturing, regional distributors, and online procurement channels determines total procurement lead times and the availability of aftermarket services.
Competitive positioning and partnership strategies that separate product-centric innovators from service-led suppliers and determine long-term market resilience
Leading suppliers in the ultrasonic oxygen flow meter space differentiate along several axes: measurement performance and certification, software and connectivity, aftermarket services, and regional fulfillment. Some firms concentrate on delivering best-in-class measurement accuracy and sector-specific approvals that satisfy healthcare and hazardous-area industrial requirements. Others invest heavily in instrument platform software, enabling edge analytics, secure telemetry, and lifecycle firmware management that enhance device value beyond the sensor itself.Partnerships and channel strategies further define competitive positions. Companies that cultivate deep OEM relationships or that integrate tightly with system integrators enjoy long-term revenue streams through bundled procurement and service agreements. Conversely, those that pursue broad distribution networks and digital channels gain faster market penetration in field-service and portable applications. The ability to support certification testing, local recalibration, and warranty services in key regions is a strong differentiator for organizations competing for large industrial and institutional accounts.
Technology roadmaps increasingly feature open communications protocols and modular firmware architectures that facilitate third-party analytics and longer device service lives. Firms that make strategic investments in these areas, while maintaining disciplined quality systems and transparent compliance documentation, are better positioned to meet complex customer requirements and withstand policy-driven supply-chain disruptions.
Practical and strategic moves for vendors and buyers to improve resilience, accelerate deployment, and monetize service-led value in instrument portfolios
Industry leaders should prioritize a coordinated set of tactical and strategic actions to secure competitive advantage. First, invest in modular hardware and firmware architectures that reduce dependence on single-source components and facilitate in-field upgrades. This reduces supply chain exposure and extends product lifecycles while preserving revenue from service offerings. Second, develop a clear interoperability and cybersecurity posture so devices can be readily integrated into existing automation stacks and meet increasingly strict data-handling expectations.Third, strengthen regional service footprints and partner networks to ensure rapid calibration and warranty support, especially in regions where local presence is a decisive procurement criterion. Fourth, expand product portfolios along two complementary axes: ruggedized fixed systems for mission-critical industrial applications and simple-to-deploy portable instruments for field diagnostics and safety inspections. Fifth, offer differentiated commercial models such as performance-based service agreements and calibration-as-a-service to align vendor incentives with customer uptime and accuracy objectives.
Finally, incorporate trade-policy scenario planning into procurement and sourcing strategies, and document alternative suppliers and assembly pathways to maintain continuity. These recommendations, pursued in parallel, will increase revenue stability, reduce operational risk, and strengthen customer retention in a market defined by technical detail and evolving compliance obligations.
A multi-source research framework combining stakeholder interviews, technical validation, and regulatory cross-checks to generate actionable strategic insights
This analysis synthesizes primary interviews with procurement leaders, engineering managers, and service providers, combined with secondary technical literature, regulatory guidance, and observed commercial behaviors across multiple regions and application domains. Primary insights were gathered through targeted discussions that focused on procurement criteria, technical trade-offs, and post-sale service expectations. Secondary inputs included standards documentation, technical white papers, and product literature to validate claims around measurement principles, certification pathways, and installation modalities.The research methodology emphasizes triangulation: corroborating qualitative interview findings with documented technical specifications and observable commercial activity to reduce bias. Careful attention was given to regulatory frameworks and certification standards relevant to medical, industrial, and safety applications to ensure compliance implications were accurately represented. Regional market behavior was assessed through a combination of supply-chain visibility, distributor engagement norms, and common procurement modalities to ensure recommendations are operationally grounded.
Limitations of the approach are acknowledged; specifically, proprietary contract terms and confidential supplier cost structures were not accessible, and real-time tariff implementations were interpreted using publicly available trade policy data and stakeholder perspectives. Despite these constraints, the methodology provides a robust basis for strategic decision-making by focusing on verifiable technical differentials, channel behaviors, and service models that materially affect adoption and lifecycle economics.
Closing synthesis highlighting why technical design, serviceability, and compliance now determine competitive advantage in flow metering rather than component cost alone
In summary, ultrasonic oxygen flow meters are transitioning from specialized instruments to strategic assets that support operational resilience, regulatory compliance, and data-driven process improvement across multiple sectors. Advances in embedded analytics, communications, and non-intrusive measurement have reduced the friction of deployment while increasing the expectations placed on vendors for ongoing software and service support. Market dynamics are being shaped by region-specific regulatory priorities, evolving procurement behaviors, and supply-chain uncertainties linked to trade policy.Successful organizations will align product development with modularity and interoperability, expand regional service capabilities, and offer commercial models that convert technical performance into measurable operational outcomes. From a buyer’s perspective, emphasis should be placed on supplier transparency, documented capabilities for calibration and certification, and the vendor’s ability to support integration into broader monitoring ecosystems. Ultimately, the value of ultrasonic oxygen flow meters is realized not only through measurement accuracy but through the data, service continuity, and regulatory assurances they enable.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- ABB Ltd.
- Aichi Tokei Denki Co., Ltd.
- Badger Meter Inc.
- Baker Hughes Company
- Brooks Instrument GmbH
- Cubic Sensor and Instrument Co., Ltd
- Dwyer Instruments Ltd.
- Emerson Electric Co.
- Endress+Hauser Group Services AG
- Faure Herman
- FLEXIM Instruments GmbH
- Fuji Electric Co., Ltd.
- HAWK Measurement Systems
- Honeywell International Inc.
- ifm electronic gmbh
- Itron Inc.
- KOBOLD Messring GmbH
- KROHNE Group
- OMEGA Engineering Inc.
- Pulsar Measurement
- Sensirion
- SICK AG
- Siemens AG
- SONOTEC GmbH
- Yokogawa Corporation of America
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
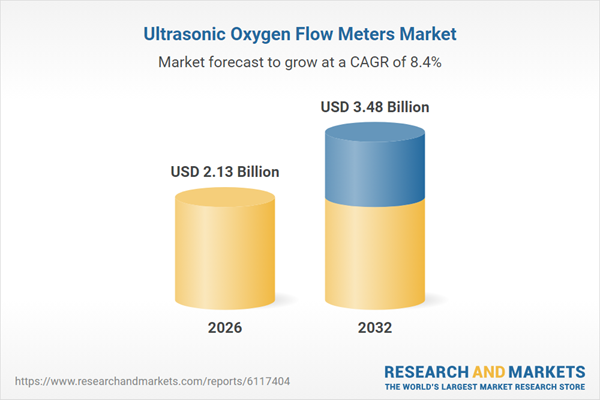
| Estimated Market Value ( USD | $ 2.13 Billion |
| Forecasted Market Value ( USD | $ 3.48 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |