Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to the clinical role, engineering evolution, and strategic pressures shaping over-the-wire balloon catheter adoption across interventional specialties
The over-the-wire balloon catheter category remains a core enabling technology for interventional procedures spanning cardiovascular, peripheral vascular, neurovascular, urology, and gynecology specialties. Clinicians rely on these devices for lesion preparation, vessel dilation, and targeted therapies where precise control over catheter positioning and balloon behavior determine procedural success. In recent years, incremental advances in materials, tip profiles, and shaft design have expanded the clinical envelope for OTW devices, enabling lower-profile access, improved deliverability in tortuous anatomy, and compatibility with adjunctive therapies such as drug delivery and neurointerventional devices.These performance improvements coincide with shifts in care delivery that prioritize outpatient and ambulatory pathways, demand lower complication rates, and seek demonstrable reductions in procedure time. From a technology perspective, developers are converging on integrated design philosophies that pair balloon compliance characteristics with catheter shaft engineering to balance pushability, trackability, and radiopacity. At the same time, regulatory attention on device labeling, sterilization, and evidence of real-world effectiveness has increased the burden on manufacturers to produce robust clinical data packages. Consequently, the competitive dynamic now rewards firms that combine engineering excellence with efficient clinical evidence generation and scalable distribution models.
How evolving clinical pathways, materials innovation, regulatory scrutiny, and distribution diversification are jointly redefining competitive priorities for over-the-wire balloon catheter manufacturers
The landscape for over-the-wire balloon catheters is being reshaped by multiple transformative forces that interact across clinical practice, technology development, and commercial strategy. Clinically, the migration toward less invasive pathways and the growth of ambulatory surgical centers alter procedural volume distribution and influence demand for low-profile, easy-to-deploy OTW systems. Technological trends emphasize materials innovation, with next-generation polymers and coating technologies improving compliance control and minimizing vessel trauma during dilation. Simultaneously, device manufacturers are increasingly integrating imaging-compatible markers and aiming for compatibility with adjunctive therapies to support hybrid procedural workflows.Operationally, supply chain optimization and strategic partnerships are becoming critical as manufacturers seek to reduce lead times and ensure consistent quality across complex component sourcing. Regulatory environments are also shifting, with enhanced post-market surveillance expectations and greater scrutiny of labeling claims, which in turn raises the bar for clinical evidence and real-world performance monitoring. Commercially, distribution strategies are fragmenting: direct sales remain important for high-touch hospital channels while distributors and digital channels expand access to ambulatory and specialty clinic settings. Taken together, these shifts reward firms that can execute cross-functional alignment between R&D, clinical affairs, and commercial teams to accelerate adoption and sustain long-term growth.
Assessment of how cumulative United States tariff measures through 2025 have increased supply chain complexity, influenced sourcing strategy, and altered pricing and procurement dynamics for device manufacturers
Cumulative tariff actions enacted by the United States through 2025 have introduced meaningful complexity into the global supply calculus for manufacturers and purchasers of over-the-wire balloon catheters. The incremental duties on key inputs and finished goods have increased landed costs for firms reliant on overseas manufacturing and component suppliers, prompting reassessment of sourcing strategies. As a result, procurement leaders and operations teams have initiated dual-track responses: some are accelerating nearshoring and multi-sourcing to reduce exposure to single-country risk, while others are renegotiating supplier agreements and redesigning components to substitute tariff-sensitive materials.These adjustments affect numerous commercial considerations. Pricing strategies must now reflect not only production and distribution expenses but also the potential volatility introduced by trade policy. Clinical adoption timelines can be influenced where cost pressures affect contract negotiations with hospital systems and ambulatory providers that operate under constrained budgets. Moreover, the tariff environment has elevated the importance of regulatory and customs expertise within corporate functions to manage classification, ensure compliance, and capture available relief mechanisms. In sum, the tariff landscape in 2025 has catalyzed operational resiliency planning and accelerated decisions around manufacturing footprint and commercial channel alignment.
Actionable segmentation perspective linking end-use care settings, procedural applications, product compliance types, and evolving distribution pathways to inform R&D and commercial prioritization
Segmentation analysis of the over-the-wire balloon catheter space highlights nuanced demand drivers across clinical settings, therapy applications, product design, and channels to market. End use segmentation demonstrates that ambulatory surgical centers increasingly demand low-profile, efficient OTW systems that enable short-stay procedures, hospitals continue to require high-performance devices for complex cases and hybrid interventions, and specialty clinics seek solutions optimized for repeatable, procedure-specific workflows. When considering application segmentation, the spectrum ranges from coronary interventions that emphasize precise compliance control and radiopacity to gynecologic applications where device compatibility with unique anatomies is critical; neurovascular use cases prioritize ultra-low-profile access and exceptional navigability, while peripheral interventions-spanning carotid arteries, lower limb interventions, and renal arteries-require a balance of balloon robustness and deliverability; urology applications further expand requirement sets to include biocompatibility and device longevity in dynamic luminal environments.Product type segmentation differentiates devices by compliance characteristics, with compliant balloons favored where vessel-conforming dilation is needed, non-compliant balloons selected for precise lumen sizing and controlled expansion, and semi-compliant platforms offering a middle ground for mixed clinical objectives. Distribution channel segmentation reveals that direct sales are preferred when clinical training and integrated service models are essential, distributors provide breadth and regional reach especially for smaller hospital networks and specialty clinics, and online sales are emerging as a supplementary channel for consumables, complementary devices, and inventory replenishment. Integrating these dimensions helps clarify which product configurations, evidence packages, and commercial approaches align with specific end-user needs and procedural trends.
Strategic regional intelligence describing how the Americas, Europe Middle East & Africa, and Asia-Pacific each shape regulatory, reimbursement, and distribution strategies for interventional catheter portfolios
Regional dynamics exert significant influence on adoption patterns, regulatory pathways, and supply chain design for OTW balloon catheters. In the Americas, demand is shaped by a mature clinical ecosystem that values evidence-driven adoption, cost containment pressures from payers, and a trend toward outpatient procedural pathways that favor devices optimized for efficiency and safety. Across Europe, Middle East & Africa, stakeholders navigate more heterogeneous reimbursement regimes and regulatory frameworks, which places a premium on adaptable market entry strategies, harmonized clinical data, and localized distribution partnerships to address wide-ranging hospital procurement norms and variable private market penetration. The Asia-Pacific region is characterized by diverse growth engines, differing levels of clinical infrastructure, and an increasing emphasis on domestic manufacturing capacity; manufacturers seeking traction there must reconcile global device standards with local regulatory requirements and competitive dynamics driven by cost-sensitive procurement.These regional distinctions also inform supply chain and commercial decisions. Manufacturers must balance centralized manufacturing efficiencies against regional regulatory certifications and tariff exposures, and they often deploy hybrid distribution strategies that combine direct presence in core markets with distributor relationships and local service support. Clinical training programs and evidence dissemination should likewise be tailored to regional clinician practice patterns and reimbursement realities to optimize adoption and long-term utilization.
Competitive architecture snapshot explaining how incumbents, specialists, and system integrators leverage scale, innovation, and service models to sustain differentiation in over-the-wire catheter portfolios
Competitive dynamics in the OTW balloon catheter arena are being defined by a mix of established medical device companies, specialist interventional device firms, and agile early-stage innovators. Incumbent players often leverage scale advantages in manufacturing, regulatory experience, and broad commercial footprints to sustain supply reliability and clinician relationships. Meanwhile, smaller and mid-sized firms differentiate through focused technology bets such as novel polymer formulations, advanced coating chemistries, or platform designs that improve deliverability in challenging anatomies. Partnerships between component suppliers, contract manufacturers, and OEMs are increasingly common, enabling faster prototyping and flexible production scaling while allowing OEMs to concentrate investment on clinical evidence and market access.Investor attention and strategic acquisitions continue to prioritize firms with demonstrated clinical differentiation and the ability to integrate into existing procedure ecosystems. At the same time, service models that combine device supply with procedural training, physician support, and data-driven outcomes tracking are gaining traction as points of competitive differentiation. Companies that can harmonize regulatory strategy, IP protection, and clinical evidence generation while managing cost-to-serve effectively are best positioned to maintain momentum as procedural practices evolve and new therapeutic applications emerge.
Practical, prioritized recommendations for executives to align R&D, supply chain resilience, clinical evidence, and channel strategy to accelerate adoption and protect margins
Industry leaders should pursue a set of prioritized actions that link product innovation to operational resilience and commercial execution. First, align engineering roadmaps to clinical pain points by investing in low-profile shaft systems, targeted balloon compliance options, and adjunct compatibility that directly address the needs of coronary, neurovascular, peripheral, gynecologic, and urology procedures. Second, restructure supply chain and sourcing strategies to reduce tariff exposure and improve responsiveness by pursuing dual-sourcing, nearshoring options for critical components, and long-term strategic agreements with key suppliers. Third, strengthen clinical evidence strategies with pragmatic trial designs and real-world evidence collection that accelerate credentialing and reimbursement dialogues with hospitals and ambulatory centers.Fourth, refine commercial channels by matching distribution models to end-use settings: deploy direct sales and clinical training in high-acuity hospital environments while leveraging distributors and digital platforms to capture ambulatory and specialty clinic demand. Fifth, invest in regulatory and customs expertise to optimize tariff classification and capture available relief mechanisms while ensuring compliance across jurisdictions. Finally, develop integrated service offerings-combining device supply, clinician education, and outcomes analytics-to create sticky relationships with health systems and differentiate beyond product features. Taken together, these actions will help organizations translate technological capability into measurable clinical and commercial outcomes.
Transparent multi-method research approach combining clinician interviews, device technical appraisal, regulatory review, patent landscaping, and supply chain diagnostics to ensure actionable conclusions
The research approach underpinning this analysis combines primary stakeholder engagement, device-level technical review, regulatory and patent landscape mapping, and supply chain diagnostics to produce a comprehensive understanding of device performance, commercial channels, and operational risk. Primary research included structured interviews with interventional clinicians, procurement leads, and device development executives to capture real-world procedural preferences, purchasing criteria, and adoption barriers. These qualitative inputs were triangulated with technical assessments of device design, materials selection, and performance claims to evaluate differentiation and potential failure modes.Regulatory dossiers, clinical trial registries, and peer-reviewed literature were systematically reviewed to confirm indications, labeling trends, and evidence generation strategies. Patent landscaping and IP review informed competitive positioning and innovation trajectories. Supply chain diagnostics assessed component sourcing, manufacturing footprints, and tariff exposure to model resilience options. Finally, synthesis workshops integrated these streams into actionable insights and scenario-based recommendations for R&D prioritization, commercial alignment, and operational planning.
Concluding synthesis that ties technological advancement, regulatory reality, and commercial discipline into a cohesive roadmap for stakeholders navigating the evolving OTW catheter landscape
The synthesis of clinical, technological, regulatory, and commercial perspectives yields a clear imperative: manufacturers and stakeholders must accelerate integrated strategies that tie product design to clinical workflows while shoring up supply chain resilience and evidence generation. Advances in material science and design are expanding the capabilities of OTW balloon catheters, enabling more precise and less invasive interventions across a wider set of clinical applications. At the same time, policy and trade dynamics require operational agility and informed procurement negotiations to mitigate cost volatility.Executives should therefore prioritize cross-functional programs that align R&D, clinical affairs, regulatory, and commercial teams to reduce time-to-clinic and increase the probability of sustained adoption. Investing in outcome-focused evidence generation, flexible manufacturing, and targeted channel strategies will help translate device innovations into enduring clinical utility and commercial viability. The path forward rewards those who act deliberately to balance technical differentiation with practical deployment considerations.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China OTW Balloon Catheter Market
Companies Mentioned
- Abbott Laboratories
- Acrostak Corp
- Alvimedica
- AngioDynamics, Inc.
- Asahi Intecc Co., Ltd.
- B. Braun Melsungen AG
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- BVM Medical Limited
- Cook Group Incorporated
- iVascular SLU
- Johnson & Johnson
- Medtronic plc
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- OrbusNeich Medical Company Limited
- QT Vascular Ltd.
- SIS Medical AG
- Teleflex Incorporated
- Terumo Corporation
- Vascular Solutions, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
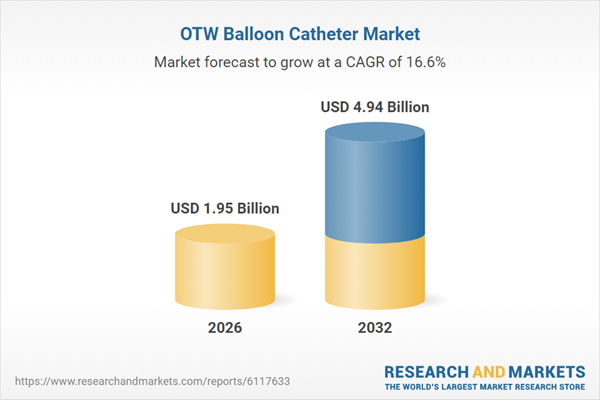
| Estimated Market Value ( USD | $ 1.95 Billion |
| Forecasted Market Value ( USD | $ 4.94 Billion |
| Compound Annual Growth Rate | 16.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |