Speak directly to the analyst to clarify any post sales queries you may have.
A clear, mechanism-focused introduction that sets the clinical, regulatory, and commercial context for IL-17 inhibitor therapies in modern dermatology and rheumatology
The advent of IL-17 pathway therapeutics has fundamentally reshaped treatment paradigms for immune-mediated dermatological conditions, with these agents redefining expectations for efficacy, onset of action, and patient quality of life. This introduction positions IL-17 inhibitors within the broader therapeutic ecosystem, highlighting how scientific advances in immunology translated into targeted biologic designs have led clinicians to reconsider long-standing care algorithms. As such, the narrative begins with the mechanism-driven rationale for IL-17 blockade, explaining how selective inhibition of IL-17A or its receptor modulates downstream inflammatory cascades central to plaque psoriasis and associated arthropathies.Moving from mechanism to clinical impact, the section explains how differences in molecular targets and delivery regimens influence real-world adoption among diverse patient populations. It situates the discussion within evolving regulatory expectations and reimbursement frameworks, noting how safety monitoring, contraindications, and long-term efficacy data shape prescriber confidence. In addition, it outlines how patient preferences-particularly for rapid symptom control and convenient dosing intervals-interact with healthcare system priorities to determine uptake. The introduction closes by framing the subsequent sections: a deep-dive into market dynamics, segmentation-driven insights, regional nuances, competitive behavior among developers and manufacturers, and pragmatic recommendations for stakeholders seeking to navigate this sophisticated therapeutic class.
An incisive review of structural shifts across clinical practice, commercialization strategies, and regulatory pathways that are reshaping IL-17 inhibitor adoption
The IL-17 inhibitor landscape has entered a transformative phase driven by convergence of scientific validation, evolving clinical practice patterns, and strategic commercialization choices. At the clinical level, accumulation of long-term safety data and head-to-head comparative evidence has encouraged prescribers to refine patient selection, optimize sequencing with other mechanism classes, and adopt shared decision-making models that foreground speed of response and durability. These clinical refinements have, in turn, influenced formulary decisions and clinical pathway updates in health systems seeking predictable outcomes and cost-effective management.Commercially, manufacturers have shifted from broad brand-building campaigns to targeted value propositions that emphasize payer-supported outcomes and adherence-friendly dosing. This strategic pivot is complemented by enhancements in patient support programs, digital adherence tools, and integrated service models that bridge specialist care and home administration. Regulatory and supply chain adaptations have also been notable; expedited review pathways for pivotal indications and investments in biologics manufacturing capacity have reduced time-to-therapy for prioritized patient cohorts. Looking ahead, convergence around biomarkers and precision prescribing is likely to accelerate, while cross-sector collaborations will reconfigure how novel indications and combination regimens are evaluated and rolled out. In this way, the landscape is not merely evolving but undergoing structural shifts that reframe competitive positioning and access strategies.
A pragmatic assessment of how tariff changes and trade dynamics can influence biologics supply chains, procurement strategies, and access to IL-17 therapies
Tariff policy adjustments and trade-related frictions can materially affect the economics and logistics of biologic therapies. In a landscape where active pharmaceutical ingredients, monoclonal antibody production inputs, and finished biologics cross borders multiple times, alterations in tariff regimes introduce cost variability and supply-chain complexity. Manufacturers and distributors must therefore adopt anticipatory sourcing strategies, reassess contract manufacturing footprints, and consider onshoring or regionalizing critical production capabilities to mitigate exposure to tariff-induced cost pressures.In parallel, payers and procurement bodies may respond to increased import costs by tightening reimbursement criteria, favoring local suppliers, or negotiating volume-based agreements that shift pricing dynamics. For healthcare providers, tariff-driven price volatility can complicate formulary planning and outpatient clinic budgeting, influencing which biologics are preferred for initiation and maintenance. Strategic responses include diversifying supplier bases, increasing vertical integration with domestic production partners, and deploying risk-sharing agreements that align cost with clinical outcomes. Ultimately, while tariffs represent a macroeconomic variable outside clinical control, their ripple effects require integrated commercial, supply-chain, and policy engagement to preserve patient access and maintain predictable therapy delivery.
Comprehensive segmentation insights revealing how product, indication, channel, regimen, dosing cadence, and patient demographics combine to shape IL-17 therapy demand and delivery
Segmentation-driven analysis provides the clearest view of demand drivers and service model requirements across the IL-17 inhibitor class. When reviewed by product, attention centers on the distinct pharmacologic profiles and administration characteristics of the three principal IL-17 agents, which inform positioning and lifecycle tactics. Based on indication, clinical pathways differ meaningfully between plaque psoriasis and psoriatic arthritis, with care teams for each indication prioritizing separate endpoints-dermatologic clearance versus joint function and structural preservation-shaping trial design, labeling language, and post-approval evidence generation.Distribution channel segmentation highlights divergent operational needs: hospital pharmacies manage inpatient initiation and complex monitoring, online pharmacies facilitate home delivery and adherence support across geographies, and retail pharmacies-both chain and independent-serve as critical touchpoints for chronic dispensing, patient education, and community-based therapy continuity. End user distinctions likewise influence service models, as home care settings demand robust patient training and remote monitoring capabilities, hospitals integrate IL-17 agents within multidisciplinary care plans, and specialty clinics create concentrated expertise that optimizes initiation and titration.
Therapeutic regimen segmentation underscores the contrast between monotherapy strategies and combination approaches that may include traditional agents such as cyclosporine or methotrexate, affecting safety surveillance and co-administration logistics. Dosing frequency segmentation-biweekly versus monthly-intersects with adherence engineering and patient preference workstreams. Finally, patient type segmentation delineates adult versus pediatric care pathways, where pediatric categories subdivide into adolescents and younger children, each requiring tailored dosing, safety monitoring, and caregiver engagement. Collectively, these segmentation lenses reveal where clinical, commercial, and operational investments generate the greatest returns.
Key regional dynamics and access considerations across the Americas, Europe Middle East & Africa, and Asia-Pacific that shape IL-17 therapy commercialization and uptake
Regional dynamics materially alter how IL-17 inhibitors are accessed, adopted, and commercialized, with each macro-region presenting distinct regulatory, payer, and clinical practice textures. In the Americas, adoption has been influenced by a robust specialist network, strong payer-driven formulary negotiation practices, and an emphasis on outcomes-based contracting; these factors drive manufacturers to prioritize real-world evidence generation and patient support infrastructures. Supply chain considerations in the Americas also incentivize flexible distribution models that can respond to both urban specialty centers and dispersed community settings.Europe, Middle East & Africa presents a mosaic of reimbursement philosophies, regulatory environments, and healthcare delivery models. Centralized pricing negotiations coexist with country-level access pathways, and payers often require comparative effectiveness data to favor inclusion. Manufacturers operating in this macro-region must balance harmonized clinical dossiers with tailored HTA submissions, while also accounting for logistical challenges in regions with fragmented infrastructure.
Asia-Pacific encompasses highly varied markets, from mature regulatory systems with private payer influence to emerging markets with rapid capacity expansion. Local manufacturing partnerships, tiered pricing strategies, and culturally attuned patient support programs are central to successful commercialization. Additionally, regulatory acceptance of external clinical data versus imperative for local clinical trials can determine time-to-reimbursement. Across regions, strategic alignment of evidence generation, pricing policy, and distribution architecture remains critical to translating clinical value into sustainable access.
Strategic competitive insights outlining how clinical differentiation, partnerships, and patient support models determine company positioning in the IL-17 inhibitor arena
Competitive behavior among companies in the IL-17 space is defined by a mix of product differentiation, evidence generation, and strategic partnerships. Leading biopharmaceutical organizations emphasize head-to-head clinical data, expanded indication pursuits, and lifecycle management tactics that reinforce clinical positioning. These tactics are complemented by investments in patient support platforms, adherence solutions, and digital ecosystems designed to lower friction at initiation and sustain long-term therapy engagement.Consequently, alliances between developers, contract manufacturers, and specialty distributors are increasingly common, enabling scale-up of biologics production while preserving commercial agility. Manufacturers also calibrate pricing and value dossiers to accommodate payer emphasis on long-term outcomes, often structuring rebate frameworks or outcome-based agreements to align with health system priorities. Emerging biotech companies contribute innovation through novel constructs, next-generation delivery formats, or combination approaches that may disrupt incumbent strategies. From a competitive standpoint, attention centers on robustness of clinical differentiation, strength of real-world evidence programs, and operational excellence in supply continuity and patient access delivery.
Actionable recommendations for pharmaceutical leaders to align evidence generation, distribution resilience, and payer engagement for sustainable IL-17 therapy adoption
Industry leaders can take several practical steps to convert clinical strengths into sustained competitive advantage while preserving patient access and system value. First, prioritize generation of comparative effectiveness and long-term safety data that directly address payer and clinician decision criteria, thereby reducing uncertainty and facilitating favorable formulary outcomes. Complement that evidence base with robust real-world studies and registries that capture adherence, quality-of-life, and health economic endpoints to support value conversations.Second, invest in distribution and service models that reduce initiation friction: streamline hospital onboarding processes, partner with online and retail channels to ensure reliable home delivery, and develop clear protocols for community-based monitoring. Third, de-risk supply chains by cultivating regional manufacturing partnerships, diversifying contract manufacturers, and implementing inventory strategies that can absorb trade policy fluctuations. Fourth, tailor patient support programs to demographic needs, offering adolescent-focused education materials, caregiver training for younger pediatric populations, and digital adherence tools aligned with dosing cadence.
Finally, engage proactively with payers and health technology assessors to pilot innovative contracting mechanisms and risk-sharing agreements that reward clinical outcomes. By integrating evidence generation, channel optimization, supply resilience, and payer-aligned commercial models, industry leaders can enhance uptake while ensuring equitable patient access across diverse healthcare contexts.
A transparent, multidisciplinary research methodology combining primary stakeholder interviews, literature review, and scenario testing to validate IL-17 inhibitor insights
The research methodology underpinning this analysis combines multidisciplinary inputs to ensure rigor, reproducibility, and relevance to decision-makers. Primary research included structured interviews with clinical specialists, pharmacy directors, payer representatives, and commercial leaders to validate clinical practice patterns, distribution constraints, and reimbursement drivers. Secondary research comprised a systematic review of peer-reviewed clinical literature, regulatory approvals and label indications, public policy documents, and guidance from professional societies to triangulate evidence on safety, efficacy, and recommended use.Analytical techniques applied include comparative clinical pathway mapping, segmentation analysis across product, indication, distribution, end user, therapeutic regimen, dosing frequency, and patient type, and scenario-based supply-chain stress testing to evaluate tariff and trade sensitivities. Regional analyses integrated regulatory pathway evaluations and payer behavior models for the Americas, Europe Middle East & Africa, and Asia-Pacific. All findings were subjected to peer review by subject-matter experts and cross-checked for consistency with recent clinical guidance and regulatory communications. Where gaps in public data existed, the methodology prioritized primary stakeholder validation and conservative interpretation to maintain analytical integrity.
A conclusive synthesis connecting clinical value, commercial strategy, and operational resilience to inform practical decisions in the IL-17 inhibitor ecosystem
The conclusion synthesizes the core implications of the IL-17 inhibitor landscape for clinical practice, commercial strategy, and policy engagement. Clinically, IL-17 blockade represents a durable, mechanism-driven option that has shifted expectations for speed and extent of disease control in plaque psoriasis and has meaningful implications for joint outcomes in psoriatic arthritis. Commercially, differential product attributes, dosing cadence, and service models are now central levers for adoption, and companies that align evidence generation with payer needs will secure preferable positioning.Operationally, supply-chain resilience and adaptive distribution strategies are essential to counterbalance macroeconomic and trade-related volatility, while segmentation-focused investments-spanning hospital initiation protocols, online delivery, retail pharmacy engagement, and pediatric-tailored approaches-unlock patient-centric pathways to therapy. Ultimately, stakeholders who integrate rigorous comparative evidence, targeted channel strategies, and proactive payer collaboration will be best positioned to translate scientific advances into accessible, sustainable care for patients living with immune-mediated dermatologic and musculoskeletal disease.
Table of Contents
20. ResearchStatistics
21. ResearchContacts
22. ResearchArticles
23. Appendix
Companies Mentioned
- AbbVie Inc.
- Almirall, S.A.
- Amgen Inc.
- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Celgene Corporation
- Eli Lilly and Company
- Galderma S.A.
- Johnson & Johnson
- LEO Pharma A/S
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- UCB S.A.
- Valeant Pharmaceuticals International, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
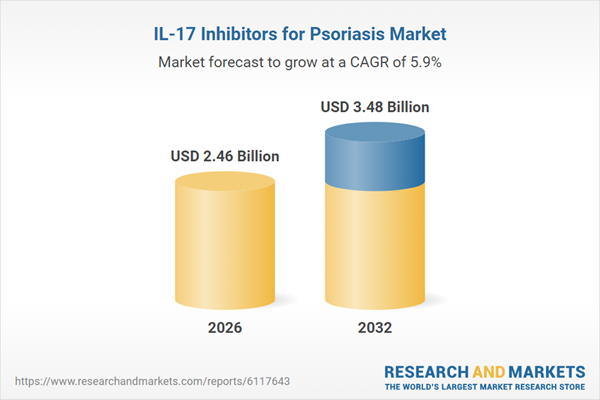
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 2.46 Billion |
| Forecasted Market Value ( USD | $ 3.48 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |