Speak directly to the analyst to clarify any post sales queries you may have.
A strategic and clinically grounded overview of complement inhibition that connects molecular innovation with evolving clinical practice and healthcare delivery trends
Complement inhibition has emerged as a pivotal therapeutic strategy across a growing set of rare and immune-mediated conditions, driven by advances in molecular biology and a deeper understanding of innate immunity pathways. The complement cascade, once considered a niche pharmacologic target, now underpins treatments for diverse indications spanning hematology, neurology, and nephrology. Scientific progress has produced multiple modalities-antibodies, peptides, RNA interference agents, and small molecules-each offering distinct pharmacokinetic and pharmacodynamic profiles that expand clinician options and create differentiated value propositions for patients.Clinical development in this space focuses not only on efficacy but on safety, durability of response, and route of administration, reflecting patient and provider demand for less disruptive therapies that support quality of life. Regulatory scrutiny and real-world evidence generation have become central to post-approval life cycle strategies. At the same time, commercial considerations including distribution channels, outpatient administration, and home-based care models are reshaping uptake dynamics. Taken together, these trends position complement inhibitors as a rapidly maturing therapeutic area where scientific innovation intersects with evolving care delivery and reimbursement ecosystems.
An in-depth exploration of how modality innovation, regulatory evolution, and value-based care models are reshaping clinical pathways and commercial dynamics in complement inhibition
The landscape for complement inhibitors is undergoing transformative shifts characterized by modality diversification, richer clinical differentiation, and reconfigured pathways to patient access. Novel modalities are augmenting the established monoclonal antibody paradigm, introducing peptide-based complement modulators, RNA interference therapeutics targeting complement component synthesis, and orally bioavailable small molecules that enable chronic disease management outside infusion settings. These therapeutic innovations bring new considerations for dosing frequency, monitoring, and safety surveillance, and they are catalyzing a shift toward more individualized treatment algorithms.Regulatory frameworks are evolving in parallel, with regulators increasingly receptive to robust real-world evidence and adaptive licensing approaches for rare disease indications, enabling faster translation of promising agents while maintaining safety oversight. Concurrently, market structures are changing as payers and providers prioritize value-based care arrangements that account for reduced hospitalization, decreased infusion burden, and improved patient-reported outcomes. Technology-enabled care models-telemedicine, remote monitoring, and digital adherence tools-are facilitating expanded use of subcutaneous and oral complement inhibitors in home and outpatient settings. Together, these shifts are redefining clinical pathways and financial models in ways that will influence product development priorities, commercialization strategies, and long-term patient outcomes.
Rigorous analysis of how U.S. tariff changes in 2025 are influencing supply chain resilience, manufacturer strategies, and payer negotiations across the complement inhibitor ecosystem
The introduction of new tariff measures in the United States in 2025 has layered additional complexity onto the global supply chains and commercial strategies for complement inhibitors. Tariff-related increases in import costs affect both finished drug products and active pharmaceutical ingredients, prompting manufacturers to reassess sourcing strategies, distribution footprints, and inventory positioning. For therapies that rely on specialized manufacturing capabilities or supply of biologic materials, these cost pressures intensify the imperative to localize critical production steps or to secure long-term supplier agreements that insulate operations from episodic trade disruptions.Beyond manufacturing, tariffs can influence pricing negotiations with payers and procurement entities by altering baseline cost structures and by creating variability in the landed cost of therapies across regions. Health systems and specialty pharmacies may respond by accelerating adoption of alternative distribution channels or by prioritizing treatments with more favorable administration profiles that reduce inpatient utilization. Clinical trial operations can also feel indirect effects: increased costs for imported supplies and devices may necessitate protocol adjustments or geographic redistribution of trial sites to maintain fiscal feasibility. In response, leading developers are pursuing strategic manufacturing investments, optimizing supply chains for redundancy, and engaging payers early to contextualize cost implications within total cost of care frameworks. These adaptive measures aim to preserve patient access while maintaining commercial viability in a higher-tariff environment.
Comprehensive segmentation-driven insights that link modality, indication, administration route, distribution channel, and end-user dynamics to strategic development and access implications
A granular understanding of segment-specific dynamics is essential for stakeholders assessing therapeutic opportunities and operational priorities. Based on product type, the landscape comprises monoclonal antibodies, peptides, RNAi therapeutics, and small molecules. Monoclonal antibodies are examined through leading agents that have set clinical benchmarks, while peptide approaches focus on modified complement-targeting compounds that prioritize targeted inhibition with alternative administration routes. RNAi therapeutics represent a distinct approach by reducing synthesis of complement components at the hepatic level, and small molecules offer oral options that enable chronic, outpatient management. Across these modalities, differences in manufacturing complexity, cold-chain requirements, and clinical monitoring obligations shape commercialization tactics and care pathway integration.Based on indication, analyses span atypical hemolytic uremic syndrome, myasthenia gravis, neuromyelitis optica spectrum disorder, and paroxysmal nocturnal hemoglobinuria. Each indication has unique diagnostic pathways, specialist care patterns, and patient support needs: for example, atypical hemolytic uremic syndrome requires coordination between adult and pediatric nephrology services, myasthenia gravis management differentiates generalized from ocular presentations and necessitates neurologic specialty care, neuromyelitis optica spectrum disorder raises antibody-status driven treatment decisions, and paroxysmal nocturnal hemoglobinuria care encompasses both adult and pediatric paradigms. These clinical distinctions impact trial design, labeling strategies, and post-authorization evidence generation.
Based on route of administration, the options include intravenous, oral, and subcutaneous strategies. Intravenous administration remains central for clinic- and hospital-based infusion models, whereas oral agents enable home-based administration and potentially broaden chronic therapy access. Subcutaneous delivery supports clinic administration as well as self-administration pathways, with training and adherence support becoming critical determinants of real-world persistence. Based on distribution channel, stakeholders engage hospital pharmacy networks, online pharmacy models, and retail pharmacy systems. Hospital pharmacies address inpatient and outpatient dispensing while online pharmacies and direct-to-patient channels introduce new patient service models. Retail pharmacies, both chain and independent, provide community-based access and require coordination on specialty drug handling and reimbursement workflows.
Based on end user, utilization is analyzed across home care, hospitals, and specialty clinics. Home care scenarios encompass caregiver administration and self-administration, highlighting the need for patient education and remote monitoring. Hospitals deliver acute and complex care, while specialty clinics-including nephrology clinics and neurology centers-serve as focal points for diagnosis, initiation, and longitudinal management. The intersection of these segmentation lenses reveals distinct go-to-market considerations: product formulation and administration route shape clinical adoption, indication-specific care pathways influence stakeholder engagement, and distribution arrangements determine patient access and adherence support mechanisms.
A nuanced assessment of regional variations in regulatory frameworks, care infrastructure, and commercialization pathways across the Americas, Europe Middle East & Africa, and Asia-Pacific
Regional dynamics exert a powerful influence on development priorities, access strategies, and operational planning for complement inhibitors, with distinct characteristics evident across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, high-concentration centers of excellence for rare diseases and established specialty pharmacy networks enable efficient patient identification and therapy delivery, while payer frameworks emphasize outcomes-based discussions and value demonstration. Europe, Middle East & Africa presents a heterogeneous environment where centralized regulatory pathways in some jurisdictions coexist with fragmented reimbursement landscapes in others, incentivizing tailored pricing strategies and differentiated market-entry sequencing.In the Asia-Pacific region, robust clinical trial capacity, rapidly expanding healthcare infrastructure, and growing domestic manufacturing capabilities present opportunities for local partnerships and regional production scaling. Regulatory timelines and adoption curves vary widely across the region, however, necessitating country-specific regulatory engagement and market access planning. The Middle East & Africa includes pockets of advanced care alongside regions with constrained specialty capacity, which underscores the importance of capacity-building programs, clinician education, and distribution partnerships to extend access. Across regions, differences in reimbursement mechanisms, patient support infrastructure, and local manufacturing policies shape the optimal commercialization architecture and risk mitigation approaches for sponsors and service providers alike.
Insightful evaluation of corporate strategies, partnerships, and operational investments that are shaping competition and long-term positioning in the complement inhibitors sector
Competitive dynamics in the complement inhibition arena are defined by a mix of established biologic platforms and emerging disruptive modalities. Key company strategies include lifecycle management of lead assets, portfolio diversification into complementary modalities, and investments in manufacturing capacity to support global distribution. Strategic alliances and licensing partnerships continue to be a dominant route to accelerate development, access specialized capabilities, and de-risk late-stage programs. Companies are also investing in advanced analytics and real-world evidence platforms to substantiate payer value propositions and to identify subpopulations that derive the greatest therapeutic benefit.Beyond product-centric moves, corporate activities increasingly emphasize patient support ecosystems and service capabilities that reduce treatment burden, such as nurse-administered clinic services, training programs for self-administration, and digital adherence tools. Manufacturers differentiating on cost structure are pursuing manufacturing process innovations and supply chain optimizations to support competitive pricing in varied reimbursement environments. At the same time, some organizations are exploring co-development of diagnostic and monitoring tools to better align treatment initiation and escalation with biomarker-driven decision-making. Taken together, these company-level initiatives illustrate a maturation of commercial playbooks-from single-product launches toward integrated care solutions that address the full patient journey.
Practical, prioritized recommendations that align supply chain resilience, evidence generation, and patient-centric commercialization to secure sustainable advantage in complement therapy markets
Industry leaders seeking durable advantage should prioritize a set of actionable initiatives that align scientific, operational, and commercial execution. First, resilience in manufacturing and supply chains must be elevated through geographic diversification, dual-sourcing of critical inputs, and targeted investments in regional production capabilities to mitigate tariff and trade volatility. Second, clinical development plans should integrate pragmatic trial designs that reflect real-world care settings and that generate evidence valued by payers, including patient-reported outcomes and health-economic endpoints.Third, commercialization should be structured around differentiated patient access models that leverage outpatient infusion centers, specialty clinics, and direct-to-patient distribution where appropriate, while also scaling training programs for self-administration to improve adherence and quality of life. Fourth, stakeholders should build payer engagement plans early, using total cost of care narratives and outcomes evidence to support reimbursement discussions. Fifth, product teams must design flexible formulation and packaging strategies to support multiple routes of administration and to accommodate diverse distribution channels. Finally, executives should invest in partnerships with diagnostics providers, digital health vendors, and specialty pharmacies to create integrated care pathways that enhance clinical effectiveness and patient experience. Collectively, these actions will strengthen market readiness and support sustainable adoption across heterogeneous healthcare systems.
A transparent, multi-method research approach combining expert interviews, regulatory analysis, and care-pathway mapping to ensure robust, actionable insights
The research supporting this executive synthesis employed a multi-method approach to ensure robustness and stakeholder relevance. Primary data collection included structured interviews with clinical experts across nephrology, neurology, and hematology, alongside conversations with payers, specialty pharmacists, and regulatory affairs professionals. Secondary efforts encompassed systematic review of peer-reviewed literature, regulatory approval documents, clinical trial registries, and public company disclosures to triangulate therapeutic positioning, safety profiles, and administration characteristics for leading agents.Analytical techniques combined qualitative thematic analysis with cross-sectional mapping of care pathways, distribution networks, and patient support models. Supply chain and tariff impact assessments were informed by trade policy tracking and consultations with manufacturing operations specialists. Findings underwent validation through iterative expert review cycles to ensure accuracy and practical applicability. The methodology emphasized transparency in data sourcing and conservative interpretation of dynamic policy developments, with all conclusions anchored in documented practice patterns, regulatory precedents, and clinically validated safety and efficacy profiles.
A concise synthesis of scientific advances, operational realities, and policy dynamics that frames where opportunity and execution risk intersect in complement therapy development
The cumulative picture of complement inhibition reveals a therapeutic area in transition: clinically mature modalities coexist with novel mechanisms that expand treatment options and reshape care delivery. Advances in peptides, RNAi therapeutics, and oral small molecules are enabling differentiated approaches to patient management, while evolving regulatory and reimbursement environments are prompting innovators to align evidence strategies with payer expectations and real-world practice. At the same time, operational factors-manufacturing footprint, supply chain resilience, and distribution models-remain critical determinants of patient access and commercial success.For stakeholders, the imperative is clear: integrate scientific differentiation with pragmatic commercialization and policy-aware operational planning. Organizations that succeed will be those that translate mechanistic innovation into demonstrable patient benefit, build resilient and adaptable supply chains, and construct payer-facing narratives grounded in outcomes and total cost of care. The next phase of complement inhibitor development and deployment will reward cross-functional strategies that balance clinical ambition with system-level realities, enabling therapies to reach appropriate patients efficiently and sustainably.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amgen Inc.
- Apellis Pharmaceuticals, Inc.
- AstraZeneca PLC
- Biogen Inc.
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Catalyst Biosciences, Inc.
- Eli Lilly and Company
- Horizon Therapeutics plc
- Ionis Pharmaceuticals, Inc.
- Janssen Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Roche Holding AG
- Sanofi
- UCB S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
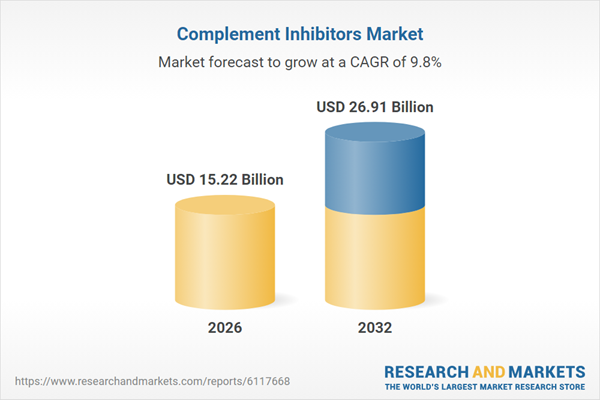
| Estimated Market Value ( USD | $ 15.22 Billion |
| Forecasted Market Value ( USD | $ 26.91 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |