Speak directly to the analyst to clarify any post sales queries you may have.
A concise framing of the clotting tube sector that situates pre-analytical device design within diagnostic reliability, supply continuity, and operational priorities
The clotting tube landscape anchors a critical element of diagnostic workflows across clinical, research, and institutional settings, where pre-analytical integrity directly affects downstream test reliability. Clotting tubes serve as the interface between patient sampling and laboratory analytics, and their material composition, additive formulations, and design configurations exert measurable influence on test performance, turnaround times, and occupational safety. This introduction frames the key technical and commercial contours of the sector, situating product characteristics within broader trends in laboratory automation, regulatory scrutiny, and supply-chain dynamics.Against a backdrop of intensifying demand for high-throughput testing and tighter quality control, manufacturers and laboratory customers are reconciling performance requirements with cost containment and sustainability goals. Innovations in tube coatings and clot activator formulations have improved sample yield and consistency, while manufacturing shifts towards scalable production of plastic alternatives have reduced breakage risk and enabled new sterilization and labeling processes. As stakeholders evaluate procurement and product development priorities, understanding how material choice, tube architecture, additive chemistry, and channel strategies interact is essential for aligning operational capabilities with clinical needs and regulatory obligations.
How automation, material innovation, additive chemistry, and digital traceability are jointly remaking pre-analytical device requirements across clinical and decentralized testing environments
The clotting tube sector is experiencing multiple transformative inflections driven by technological advances, evolving laboratory models, and heightened focus on pre-analytical integrity. Manufacturers are responding to automation demands by optimizing tube geometry, cap systems, and label compatibility to support robotic handling and barcode scanning. Concurrently, there is a measurable migration from glass to engineered plastics in many contexts, motivated by reduced breakage, lower shipping cost, and compatibility with automated centrifugation and storage systems. These material transitions are accompanied by refinements in additive chemistry, including more consistent serum clot activators and calibrated citrate concentrations that reduce variability in coagulation assays.Patient-centric and laboratory-centric shifts also shape product requirements. The expansion of point-of-care testing and decentralized sample collection has increased demand for closed-system designs that minimize contamination risk and maintain biosafety during transport. At the same time, clinical practice is placing greater emphasis on assays such as immunoassays for tumor markers and hormone profiling, which impose stricter demands on sample integrity and additive interference. Sustainability considerations are gaining traction, prompting suppliers to explore recyclable or lower-carbon materials and to optimize packaging and distribution models. Finally, digital integration-ranging from unique sample identifiers to cloud-enabled chain-of-custody records-is improving traceability and reducing pre-analytical errors, which reinforces the value proposition of higher-specification tube formats.
Assessment of how 2025 tariff measures reshaped supply chains, procurement practices, regulatory revalidation, and supplier selection dynamics across the sector
The introduction of tariff measures in the United States in 2025 has exerted pressure across procurement, manufacturing, and distribution pathways for consumable diagnostic devices. Suppliers that rely on imported raw materials such as medical-grade glass or specialized polymer resins have evaluated cost pass-through strategies, renegotiated supplier contracts, and sought alternative sourcing to preserve competitive pricing for laboratory customers. These adaptations often necessitate reconfiguration of procurement workflows and longer lead times as secondary suppliers are qualified and validated for clinical use.Distribution and channel partners have likewise adjusted inventory and logistics practices. To mitigate customs delays and duty-related cost volatility, some distributors increased safety stock or shifted to regional manufacturing partners, while direct sales teams emphasized bundled service offerings and value-added support to justify price adjustments. For laboratories and hospitals that operate under constrained budgets, procurement teams have had to balance short-term cost implications with long-term operational continuity, favoring suppliers that can demonstrate stable supply, robust quality assurance, and transparent cost structures. Regulatory compliance considerations have intersected with tariff impacts, since any change in material composition or supplier often triggers revalidation and documentation updates for clinical laboratories, thereby increasing the operational burden of switching sources.
Strategic responses observed across the sector include consolidation of supplier relationships to achieve volume discounts that offset duty impacts, investment in nearshoring of critical components to reduce tariff exposure, and development of product families that are designed to be compatible with multiple additive chemistries. These dynamics have also accelerated conversations around multi-sourcing as a resilience strategy, encouraging laboratory procurement teams to maintain validated alternatives for key consumables. While short-term disruptions have been uneven across regions and product classes, the cumulative effect has been to elevate supply chain resilience and supplier transparency as decisive criteria in purchasing decisions.
Deconstructing product strategies through material choice, tube architecture, additive chemistry, channel preference, and end-user requirements to clarify competitive positioning
Material selection remains a defining axis of product strategy, with glass retaining advantages for certain assays due to inertness and thermal stability while engineered plastics are increasingly preferred where breakage risk, weight, and compatibility with automated systems matter. Plastic tubes facilitate design innovation in cap systems and labeling integration, whereas glass formats continue to be specified where long-term chemical stability or specific diagnostic workflows demand it. Manufacturers are calibrating production lines and quality control processes to manage both material classes efficiently, thereby offering laboratories options that align with their automation level and handling protocols.Tube architecture influences clinical workflow and biosafety. Closed-system designs are favored in high-volume automated laboratories and decentralized collection environments because they reduce contamination risk and streamline robotic handling, while open systems persist in manual or specialized applications where direct operator access is necessary. The application segmentation underlines divergent performance requirements: clinical chemistry assays such as cholesterol, glucose, and uric acid demand consistent serum separation and minimal additive interference; coagulation testing-measured through activated partial thromboplastin time, prothrombin time, and thrombin time-places a premium on precise citrate buffering and volumetric accuracy; and immunoassays, encompassing hormone assays and tumor markers, require stringent control of pre-analytical variables to avoid signal suppression or enhancement.
Additive formulations differentiate product performance at a chemical level. Serum clot activators, whether based on micronized silica or silicone coatings, accelerate clot formation and influence serum clarity, which can affect downstream analyte measurement. Sodium citrate tubes, offered in 3.2% and 3.8% concentrations, must be matched to coagulation assay protocols to preserve result comparability. Sales channel strategy impacts market access and service levels: direct sales enable closer integration with large health systems and bespoke contract terms, whereas distributor networks provide breadth in regional reach and logistical support. End-user segmentation further shapes product requirements and procurement behaviors: academic and reference laboratories prioritize batch consistency and documentation for research rigor; diagnostic laboratories emphasize throughput and traceability; hospitals look for robustness and compatibility with existing systems; and research institutes often require specialized configurations and flexible supply arrangements.
Evaluating how regional regulatory regimes, manufacturing footprints, and healthcare infrastructure drive differentiated demand, supply strategies, and market access across global regions
Regional dynamics reflect distinct clinical demand patterns, regulatory environments, and manufacturing footprints. In the Americas, demand is driven by large integrated health systems, widespread adoption of automation, and a mature market for centralized diagnostic testing. Procurement tends to favor suppliers that can demonstrate consistent quality, rapid technical support, and integrated services that reduce the total cost of ownership for health systems. Regulatory oversight emphasizes conformity with accredited laboratory standards and product traceability, which elevates the importance of supplier documentation and validation support.Europe, Middle East & Africa presents a heterogeneous set of market conditions where regulatory regimes, reimbursement frameworks, and laboratory infrastructure vary significantly. Western European markets often demand higher specification products and rigorous conformity documentation, while many markets in the Middle East and Africa present growth opportunities tied to expanding diagnostic capacity and investments in hospital infrastructure. In these regions, distribution partnerships and local certification pathways are pivotal for market entry and scale, and manufacturers often develop region-specific commercial models to accommodate diverse procurement cycles and institutional capabilities.
Asia-Pacific encompasses a broad spectrum of manufacturing capability and clinical demand. Several countries in the region combine advanced laboratory networks with strong local manufacturing capacity, creating efficient supply chains that serve both domestic and export markets. Other economies within the region are expanding diagnostic access through investments in hospitals and reference centers, increasing demand for both basic and higher-specification consumables. Regulatory harmonization efforts and regional trade agreements influence sourcing decisions, while proximity to polymer and glass manufacturers can reduce lead times and logistics complexity for manufacturers and customers alike.
Insights into competitive positioning where scale, regulatory capability, regional agility, and integrated service models determine supplier differentiation and buyer preference
Competitive dynamics are defined by a mix of global manufacturers that maintain broad product portfolios and regional specialists that differentiate through agility, service, and localized supply. Leading producers invest in production scale, quality management systems, and regulatory affairs capabilities that facilitate supply to complex institutional customers. These firms often prioritize validated product families, extensive documentation, and integration support for automation and laboratory information systems. Regional and niche players focus on rapid product customization, regional certification, and cost-competitive offerings that appeal to distributed laboratory networks and budget-constrained institutions.Partnerships between reagent suppliers, device manufacturers, and laboratory service providers are increasingly common, as integrated solutions reduce adoption friction and create stickier customer relationships. Contract manufacturing organizations and third-party logistics providers contribute to capacity flexibility, while strategic alliances with distribution networks enable market penetration in geographies where direct presence is limited. Product differentiation is achieved through incremental innovation in additive formulations, cap and stopper design, and packaging solutions that preserve sample integrity during extended transport. Commercially, firms that combine technical support with managed inventory programs and training resources tend to outperform peers in institutional contracts where reliability and service continuity are prioritized.
Actionable, high-impact strategies for suppliers and buyers to enhance resilience, automation compatibility, regulatory readiness, and commercial differentiation in the sector
Industry leaders should prioritize multi-dimensional resilience strategies that blend supplier diversification, nearshoring, and validated secondary sourcing to reduce exposure to tariff-induced cost shocks and logistics disruptions. Investing in dual-sourcing arrangements for critical raw materials and establishing regional manufacturing touches can shorten lead times and reduce customs complexity. These initiatives should be paired with proactive regulatory planning so that any supplier transition or material substitution triggers rapid revalidation with minimal operational disruption.Manufacturers and distributors must accelerate product compatibility with automation and digital workflows by standardizing tube labels, cap coding, and barcode systems, while coordinating with laboratory automation vendors to certify handling characteristics. Concurrently, developing additive portfolios that address both high-throughput clinical chemistry and sensitive immunoassay requirements will expand addressable use cases. Commercial teams should refine channel strategies to balance direct engagement with large health systems and the broad reach provided by experienced distributors; value-added services such as managed inventory, training, and performance warranties will strengthen long-term customer relationships.
Sustainability and packaging optimization present both cost and reputational opportunities. Reductions in weight, improved recyclability, and concentrated logistics models lower carbon footprint while responding to buyer expectations. Finally, organizations should invest in customer education and co-development initiatives with major end users-hospitals, reference laboratories, and research institutes-to align product roadmaps with evolving assay demands and to build defensible partnerships through shared validation and quality outcomes.
Description of the mixed-methods research approach that combined primary stakeholder interviews, product evaluations, and rigorous secondary verification to ensure robust insight generation
This analysis is rooted in a mixed-methods approach that integrates qualitative interviews, product testing evaluations, document review, and secondary intelligence. Primary research included structured conversations with laboratory managers, procurement officers, quality assurance professionals, and supply chain leads to capture operational realities, pain points, and procurement criteria. Product performance observations and technical datasheets were synthesized to compare material attributes, additive chemistries, and handling characteristics across tube formats.Secondary inputs included regulatory filings, standards documentation, conference proceedings, and public company disclosures to contextualize manufacturing capability and compliance practices. Findings were triangulated across multiple data sources to reduce bias and to validate recurring themes. The research team applied rigorous criteria for source credibility, emphasizing peer-reviewed literature, official regulatory guidance, and practitioner testimony. Limitations are acknowledged: while qualitative patterns and technological trends are robust, operational specifics can vary by institution, and suppliers’ proprietary formulation details are often confidential. To mitigate these limits, the methodology emphasizes cross-verification and offers an evidence-based but practical set of conclusions and recommendations.
Synthesis of how design, additive chemistry, supply resilience, and collaborative service models collectively define competitive advantage and operational reliability in the industry
The cumulative narrative highlights a sector in active transition: technical refinements in tube design and additive chemistry, shifting material preferences, and evolving procurement imperatives are collectively reshaping how laboratories and suppliers interact. Operational resilience, automation compatibility, and regulatory documentation emerge as dominant decision criteria that influence purchase choice across end-user types. Laboratories are prioritizing suppliers that can deliver consistent quality, rapid technical support, and validated alternatives to mitigate supply chain risk, while manufacturers are balancing scale with the need for regional agility and customization.Looking forward, organizations that align product development with automation standards, invest in supply chain redundancy, and offer integrated services will be best positioned to capture demand from large institutional buyers and to support decentralized testing models. Strategic collaboration across the value chain-spanning manufacturers, distributors, automation vendors, and laboratory customers-will accelerate adoption of higher-specification products and reduce pre-analytical variability, thereby improving clinical outcomes and operational efficiency. The conclusion underscores that deliberate investments in compatibility, resilience, and customer support are the most effective levers for sustaining competitive advantage.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Clotting Tube Market
Companies Mentioned
- A.N. PHARMA
- A.V. Consumables
- ACEE Tubes Pvt Ltd
- Ajosha Bioteknik Pvt Ltd
- Apex Bio-medicals
- BD (Becton, Dickinson and Company)
- BDK Life Science
- Bio-x
- Cardinal Health Inc.
- CML Biotech Pvt Ltd
- Diagnostic Care And Trading Pvt Ltd
- Fusion Biotech
- Greiner Bio-One
- Human Biomedicals LLP
- Labtech Disposables
- Levram Life Sciences Pvt Ltd
- MK Plast
- Nasmed Diagnostics Pvt. Ltd.
- Quantum Biomedicals
- Raj Biosis Private Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
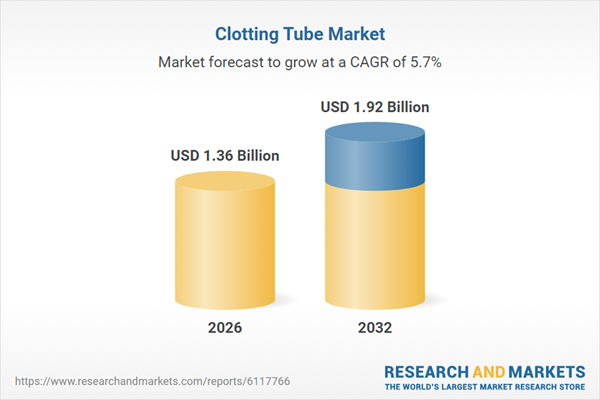
| Estimated Market Value ( USD | $ 1.36 Billion |
| Forecasted Market Value ( USD | $ 1.92 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |