Speak directly to the analyst to clarify any post sales queries you may have.
A strategic orientation to the converging technologies, clinical validation imperatives, and operational dynamics driving adoption of AI-enabled electrocardiogram systems
AI-driven electrocardiogram analysis is transforming how clinicians detect, monitor, and manage cardiovascular conditions by combining advances in sensing hardware, algorithmic interpretation, and cloud-native analytics. This introduction situates the reader within the current technological and clinical milieu, where high-frequency waveform capture from wearable patches and implantables converges with machine learning models that automate classification, feature extraction, and pre-processing. The interplay between devices and software has broadened diagnostic reach beyond traditional facility walls, enabling remote monitoring, teleconsultation, and continuous arrhythmia surveillance. Consequently, clinical workflows are evolving: frontline clinicians and remote monitoring teams increasingly rely on algorithmic triage to prioritize cases and streamline specialist referrals.Regulatory scrutiny and clinical validation have accelerated in parallel, raising the bar for demonstration of safety, accuracy, and explainability. Interoperability expectations and data governance obligations now shape procurement decisions as health systems weigh on-premise deployments against cloud-based services that promise scalability and real-time analytics. Commercial dynamics are equally fluid, with new entrants innovating at the edge while established medical device companies pursue software upgrades and services to protect installed bases. This introduction frames the executive audience to consider not only the technical capabilities of AI ECG solutions but also the operational, regulatory, and economic forces that will determine near-term adoption and long-term integration into standard care pathways.
How breakthroughs in edge inference, federated learning, robust signal processing, and evolving reimbursement are reshaping clinical pathways and commercialization models
The landscape for AI ECG analysis is shifting as breakthroughs in model architectures, sensor miniaturization, and connectivity coalesce with changing care delivery models. Edge computing and federated learning are enabling more sophisticated on-device inference while preserving patient privacy, which in turn reduces latency for critical alerts and lowers the bandwidth burden on health networks. Simultaneously, classification algorithms are becoming more robust to noisy ambulatory signals through enhanced pre-processing pipelines and feature extraction methods, increasing real-world clinical utility. These technical improvements are fostering new clinical workflows: remote monitoring programs that once focused on episodic events are expanding toward continuous risk stratification and proactive interventions.Concurrently, reimbursement environments and value-based care initiatives are incentivizing outcomes rather than episodic testing, prompting providers to integrate AI ECG analytics into chronic disease management programs. Strategic partnerships between device manufacturers, algorithm developers, and cloud service providers are accelerating commercialization and enabling bundled offerings that combine hardware, software, and managed services. Regulatory bodies are refining pathways for software-as-a-medical-device, which is enabling faster iterations while maintaining patient safety. Together, these transformative shifts create an ecosystem where clinical effectiveness, data stewardship, and operational scalability determine which solutions move from pilot projects to standard of care.
An evidence-based assessment of how recent trade policy measures and tariff-related supply chain shifts are influencing procurement, manufacturing strategies, and adoption timelines
Trade policy measures in the United States, including tariffs and export controls enacted or contemplated through 2024 and into 2025, have introduced an additional layer of complexity for manufacturers and solution providers operating global supply chains. Cumulative tariff impacts have raised component sourcing costs and prompted procurement teams to reassess vendor portfolios, particularly for hardware-intensive subsegments such as ECG devices and wearable monitors. The consequences extend beyond direct cost pressures: longer lead times for semiconductors and sensors have incentivized firms to diversify suppliers, accelerate localization of critical components, and invest in inventory strategies to buffer disruption. These adaptations influence product roadmaps and timing for clinical deployments, as organizations balance the need for feature-rich devices against the operational imperative to maintain supply continuity.At the same time, tariffs have intensified conversations around nearshoring and strategic partnerships that can mitigate exposure to single-source geographies. Software and services providers have responded by emphasizing cloud-based deployment options and modular architectures that decouple hardware dependencies where feasible. For healthcare providers, increased procurement complexity has sometimes slowed adoption cycles, as capital planning now incorporates supply-chain risk assessments. From an investment perspective, the tariff environment has made vertically integrated business models and companies with diversified manufacturing footprints more attractive to risk-conscious stakeholders. Across the value chain, the net effect has been a pragmatic shift: stakeholders are emphasizing supply resiliency, contractual flexibility, and product designs that tolerate component variation without compromising clinical performance.
A segmented synthesis connecting component architectures, end-user requirements, deployment choices, ECG modalities, and clinical applications to reveal actionable opportunity areas
A nuanced segmentation lens clarifies where value accrues and where adoption friction remains across the AI ECG ecosystem. Based on component, the market encompasses hardware, services, and software. Hardware includes ECG devices and wearable monitors that capture cardiac signals at varying fidelity and duration, while services cover implementation and maintenance offerings that ensure clinical integration and lifecycle support. Software spans classification algorithms, feature extraction modules, and pre-processing toolchains that collectively translate raw waveforms into clinically actionable insights. Based on end user, adoption patterns differ across clinics, diagnostic labs, home healthcare providers, and hospitals, with hospitals prioritizing integration with electronic health record systems and home healthcare seeking simplicity and patient-centric form factors to support at-home monitoring programs.Based on deployment mode, choices between cloud and on-premise deployments reflect trade-offs in scalability, data control, and latency requirements; cloud deployments may further subdivide into private cloud and public cloud options that influence security architecture and total cost of ownership. Based on ECG type, device and algorithm requirements vary by ambulatory ECG, event monitoring, and resting ECG; ambulatory ECG itself covers Holter monitoring, implantable loop recorders, and wearable patches that offer different monitoring durations and signal characteristics. Based on application, solutions are optimized for arrhythmia detection, myocardial ischemia identification, stress testing augmentation, and telehealth use cases; telehealth often encompasses remote monitoring and teleconsultation, with remote monitoring built upon cloud analytics and smartphone integration capabilities. Integrating these segmentation dimensions reveals where clinical value, technical differentiation, and commercial opportunity intersect, enabling targeted product strategies and go-to-market prioritization.
A regional strategic framework that aligns clinical, regulatory, and commercial imperatives across Americas, Europe Middle East & Africa, and Asia-Pacific markets to guide market entry and scaling
Regional dynamics materially shape adoption curves, regulatory expectations, and commercial strategies for AI ECG solutions. In the Americas, advanced health systems and a strong reimbursement focus on value have supported early adoption of remote monitoring programs and integration with ambulatory care models, with clinicians seeking robust clinical evidence and interoperability to justify procurement. Europe, Middle East & Africa present a heterogeneous landscape: pockets of high regulatory rigor and digital health investment coexist with markets where infrastructure constraints and variable reimbursement models require lightweight, cost-efficient solutions and flexible deployment modes. In these regions, demonstrating compatibility with regional health information exchanges and compliance with stringent data protection frameworks is often a gating factor for enterprise deals.Asia-Pacific exhibits rapid innovation in mobile health and wearables, driven by large populations, rising chronic disease burdens, and tech-forward consumer behaviors. Adoption here leans toward scalable cloud-native solutions and smartphone-integrated monitoring that can support both urban tertiary centers and remote community health programs. Across all regions, partnerships with local providers, attention to language and cultural customization of patient-facing interfaces, and a clear roadmap for regulatory compliance are critical to commercial success. Geographic strategy therefore requires blending globally competitive technology with locally tailored implementation plans that reflect procurement cycles, clinical practice patterns, and data governance regimes.
Competitive dynamics and strategic partnership trends that define how device manufacturers, software innovators, and service providers are shaping clinical adoption and enterprise procurement decisions
Industry participants span a spectrum from established medical device manufacturers and enterprise software firms to specialized AI startups and managed service providers, each bringing distinct strengths to the AI ECG ecosystem. Legacy device manufacturers contribute manufacturing scale, clinician relationships, and established regulatory pathways, which are valuable for broad hospital deployments and integrated EHR workflows. Pure-play software vendors and algorithm developers focus on model performance, explainability, and rapid iteration, often partnering with hardware vendors or cloud providers to embed their analytics within end-to-end solutions. Emerging startups frequently differentiate through niche clinical applications-such as implantable loop recorder analytics or smartphone-based arrhythmia screening-or through novel business models that bundle analytics with subscription-based monitoring services.Strategic activity across the competitive landscape has centered on partnerships, distribution agreements, and selective acquisitions that accelerate time-to-market and expand clinical validation footprints. Companies with comprehensive service offerings that combine implementation support and ongoing maintenance have an advantage when negotiating enterprise contracts. Meanwhile, cloud providers and platform companies are instrumental in scaling analytics and offering modular infrastructures that lower the barrier to clinical integration. For buyers, vendor selection increasingly depends on demonstrable clinical outcomes, interoperability credentials, and supply resilience rather than solely on individual features, elevating the importance of cross-organizational collaboration and third-party validation.
High-impact strategic moves for clinical validation, supply-chain resilience, flexible deployment, and partnership-driven commercialization to accelerate adoption and reduce operational risk
Industry leaders should pursue a coordinated strategy that aligns clinical validation, supply-chain resilience, and customer-centric commercialization. First, prioritize rigorous prospective clinical studies and post-market surveillance to demonstrate real-world effectiveness and build clinician trust; invest in model explainability and integration pathways that allow clinicians to interpret algorithm outputs and incorporate them into diagnostic decision-making. Second, diversify component sourcing and consider modular hardware designs that tolerate supplier variability to reduce exposure to tariff-induced disruptions and component shortages. Third, design flexible deployment architectures that support both private and public cloud options as well as on-premise installations to meet varied data governance and latency requirements across customers.Additionally, cultivate partnerships with payers, large provider networks, and telehealth platforms to create bundled care pathways that align incentives toward improved outcomes. Emphasize patient engagement features and smartphone integrations to enhance adherence in home monitoring programs and to capture longitudinal data that strengthens algorithm performance. Finally, establish transparent data governance policies and security certifications to address privacy concerns and streamline procurement approvals. Together, these actions will position organizations to capture clinical value, mitigate operational risk, and accelerate sustainable adoption across care settings.
A rigorous mixed-methods research protocol combining clinician interviews, regulatory validation, vendor profiling, and scenario analysis to ensure robust and actionable insights
The research approach draws on a mixed-methods framework that triangulates primary interviews, expert validation, and systematic secondary research. Primary engagement includes structured interviews with cardiologists, clinical informaticists, procurement leaders, and technology officers to capture frontline perspectives on workflow integration, evidence needs, and procurement criteria. Expert validation sessions with regulatory and data-security specialists ensure that compliance interpretations align with current guidance and that risk assessments reflect realistic implementation constraints. Secondary research synthesizes peer-reviewed clinical studies, public regulatory guidance, and vendor technical documentation to map technological capabilities and product differentiation.Data synthesis applies qualitative coding to interview transcripts and thematic analysis to identify recurring adoption barriers and value drivers. Comparative vendor profiling captures product features, deployment models, and service offerings, while scenario analysis explores supply-chain sensitivities and cross-regional regulatory permutations. The methodology incorporates iterative quality checks, double-coding of critical input, and stakeholder review cycles to ensure robustness. Limitations are acknowledged: rapid technological iteration and evolving policy environments require periodic updates, and insights reflect prevailing conditions at the time of analysis rather than long-term projections. Nevertheless, this systematic approach yields actionable intelligence grounded in clinician experience, technical validation, and regulatory context.
A concise synthesis highlighting the convergence of technological maturity, clinical validation, operational readiness, and strategic priorities that will determine adoption success
In conclusion, AI-enabled ECG analysis is at an inflection point where technological maturity, clinical demand, and commercial readiness are aligning to expand diagnostic reach and enable proactive cardiac care. Continued improvements in signal processing, algorithm robustness, and deployment flexibility will drive deeper integration into care pathways, while regulatory clarity and reimbursement alignment will determine the pace at which pilots scale into standard practice. Supply-chain resilience and deployment architecture choices will be key operational levers for manufacturers and providers navigating tariff-induced volatility and component sourcing challenges. Ultimately, those organizations that combine rigorous clinical evidence with flexible, secure deployment models and strong local partnerships will be best positioned to capture sustainable value.Decision-makers should prioritize investments that strengthen clinical validation, interoperability, and patient engagement, while also addressing practical constraints such as supply-chain risk and data governance. By doing so, stakeholders can ensure that AI ECG technologies not only augment diagnostic capacity but also deliver measurable improvements in patient outcomes and system efficiency. The path forward requires balanced attention to technical innovation, clinical credibility, and operational readiness to realize the full potential of AI-driven cardiac monitoring and analysis.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China AI ECG Analysis Tool Market
Companies Mentioned
- AliveCor, Inc.
- Bardy Diagnostics, Inc.
- Biosensics LLC
- BioTelemetry, Inc.
- CardioComm Solutions, Inc.
- Cardiologs Technologies
- EMLI-MED
- GE Healthcare
- HeartFlow, Inc.
- Impulse Dynamics
- iRhythm Technologies, Inc.
- LivaNova PLC
- Medtronic plc
- Nanowear Inc.
- Preventice Solutions
- QT Medical, Inc.
- VisCardia, Inc.
- VivaLNK, Inc.
- Zebra Medical Vision
- Zio
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
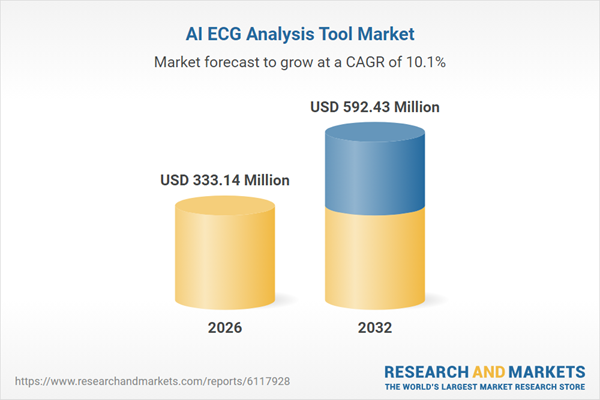
| Estimated Market Value ( USD | $ 333.14 Million |
| Forecasted Market Value ( USD | $ 592.43 Million |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |