Speak directly to the analyst to clarify any post sales queries you may have.
A forward-looking overview of how long-acting injectables and implantable platforms are redefining prevention strategies and operational priorities across health systems
Parenteral pre-exposure prophylaxis is reshaping HIV prevention by decoupling adherence from daily oral regimens and offering sustained protection through long-acting injectables and implantable platforms. This shift elevates clinical efficacy discussions into strategic conversations about supply, distribution, patient experience, and health system readiness. As clinical evidence accumulates for molecules such as cabotegravir and lenacapavir, stakeholders across industry, public health, and clinical care are recalibrating pathways for introduction, scaling, and patient access.The introduction of implantable devices-both biodegradable and non-biodegradable-alongside prefilled syringe formats has broadened the design space for PrEP delivery. These options introduce new operational requirements for manufacturing, cold chain considerations for some biologics, device sterilization standards, and post-procedure follow-up protocols. Consequently, product development teams, procurement officers, and clinic managers must integrate pharmacology, device engineering, and service delivery design to realize clinical benefits for diverse populations.
Beyond clinical and technical considerations, equity and access remain central. Direct-to-patient programs, specialty clinics, and community health centers will play instrumental roles in ensuring uptake across urban and rural settings. This introduction frames the remainder of the report by mapping how product innovation, distribution models, regulatory dynamics, and payer interactions converge to determine real-world impact and adoption trajectories.
How pharmacologic breakthroughs, device engineering, and care model innovations are collectively disrupting conventional HIV prevention workflows and stakeholder priorities
The landscape for parenteral PrEP is undergoing transformative shifts driven by converging advances in pharmacology, device engineering, and service delivery. Clinical trials demonstrating durable protection from infrequent dosing have accelerated regulatory conversations and created a pathway for broader adoption. Concurrently, innovations in implantable polymers and long-acting injectable formulations are reducing the frequency of clinical encounters required to maintain protection, which changes the calculus for patient adherence, clinic capacity, and reimbursement structures.Manufacturers are optimizing formulation stability and delivery mechanisms, while service providers are piloting hybrid care models that blend in-person procedures with home-based follow-up and telehealth check-ins. These changes expand the role of community-based sites and specialty clinics and place renewed emphasis on training, post-procedure monitoring, and integration with existing HIV prevention services. In tandem, payer and procurement stakeholders are evaluating value propositions that must capture clinical outcomes, reductions in downstream HIV-related costs, and patient-centered measures such as convenience and acceptability.
Finally, the competitive landscape is evolving: differentiated product attributes, evidence generation strategies, and channel partnerships will determine which offerings align most closely with health-system priorities. This transformation is not only technological but operational and commercial, requiring coordinated responses across the product lifecycle from R&D through patient delivery.
Understanding the multifaceted supply chain and procurement consequences stemming from 2025 tariff measures and the strategic responses that preserve access and continuity of care
The introduction of trade measures in 2025 affecting imports of medical components and polymers is creating a new variable in the supplier and cost equation for parenteral PrEP. Tariffs on key inputs such as high-performance polymers used in implantable devices, specialized syringes, and certain active pharmaceutical ingredient intermediates elevate landed costs for manufacturers and contract assemblers. In response, procurement teams are reassessing supplier diversification, nearshoring possibilities, and longer-term contracts to stabilize supply while protecting margins.Elevated import costs also ripple through pricing discussions with payers and public programs. Health-system procurement officers and manufacturers are negotiating around bundled pricing, volume-based discounts, and risk-sharing arrangements to mitigate short-term cost pressures. At the same time, manufacturers with vertically integrated capabilities or access to alternate raw material sources gain relative advantage for maintaining stable supply and competitive pricing strategies.
Operationally, tariffs are incentivizing investment in domestic production capacity and contract manufacturing relationships that reduce reliance on vulnerable cross-border supply chains. This strategic pivot carries capital and timeline implications for the industry but offers resilience against future trade volatility. For stakeholders planning introductions and scaling, the tariff environment underscores the importance of layered contingency planning for sourcing, inventory buffering, and contractual protections to preserve continuity of patient access.
Actionable segmentation intelligence that clarifies how product, molecule, distribution, end-user, and regimen variations uniquely shape clinical adoption and operational design
Segmentation drives strategic choices across clinical development, commercial strategy, and distribution design for parenteral PrEP. Product-type distinctions between implantable devices, long-acting injectables, and prefilled syringes determine regulatory pathways, procedural training needs, and device lifecycle management. Within implantable devices, the dichotomy of biodegradable polymers versus non-biodegradable polymers affects follow-up protocols and patient counseling on removal versus natural resorption. For long-acting injectables, molecule-specific profiles for cabotegravir and lenacapavir shape dosing intervals, interaction risk assessments, and monitoring schedules.Molecule-type segmentation further refines clinical positioning and formulary negotiations, with each compound presenting unique safety, efficacy, and drug-drug interaction considerations that influence prescriber preference and guideline adoption. Distribution-channel segmentation between direct-to-patient programs, hospital pharmacies, retail pharmacies, and specialty clinics frames access dynamics; direct-to-patient programs themselves bifurcate into home delivery and mail order options, while specialty clinics subdivide into community health centers and infectious disease clinics, each with different capacity and reach.
End-user segmentation highlights divergent operational requirements and outreach strategies across community health centers, HIV clinics, and research centers. Community health centers split across rural centers and urban centers, requiring tailored engagement models and logistics, while research centers differentiated into academic institutions and private research organizations serve as hubs for evidence generation and early adoption. Regimen segmentation between on-demand dosing and periodic dosing influences adherence supports and appointment cadence; on-demand approaches often divide into post-exposure-only and pre-exposure-only strategies, whereas periodic dosing typically separates into three-month interval and two-month interval schedules. These intersecting segments inform clinical protocols, go-to-market priorities, and the design of patient support services required for scalable implementation.
A region-by-region synthesis revealing how infrastructure, regulatory environments, and payer dynamics uniquely shape the rollout and sustainability of parenteral PrEP offerings
Regional dynamics significantly influence adoption pathways and implementation complexity for parenteral PrEP. In the Americas, established HIV prevention infrastructures, centralized regulatory processes in some jurisdictions, and active public procurement channels facilitate pilot programs and rapid scale-up in urban centers, while rural access remains a persistent operational challenge that requires targeted distribution strategies and DTP innovations. The Americas also feature diverse payer arrangements that influence reimbursement negotiations and the structure of value-based contracting.Europe, Middle East & Africa presents a mosaic of regulatory frameworks, infrastructure readiness, and financing models that demand tailored approaches. High-income countries in Europe may rapidly integrate long-acting options into care pathways, supported by clinician training and specialty clinic capacity. In contrast, many countries across the Middle East & Africa require investment in cold chain and procedural capacity along with coalition-based procurement to lower barriers to entry, especially for rural deployments. Public sector programs and donor partnerships can accelerate access but necessitate coordinated evidence packages and cost-effectiveness narratives.
Asia-Pacific spans mature markets with advanced manufacturing and regulatory sophistication alongside emerging economies where decentralized delivery and community health systems dominate. In higher-income markets, nearshoring and domestic production capabilities can offset trade headwinds, whereas in lower-resource settings, mobile clinics, community health center networks, and simplified periodic dosing regimens will be central to increasing reach. Across all regions, regulatory alignment on safety monitoring and standardized training protocols will be critical to realizing equitable access.
How leading developers and manufacturers are aligning clinical evidence, production resilience, and multichannel distribution partnerships to secure competitive advantages
Company-level strategies are coalescing around three core priorities: evidence differentiation, manufacturing and supply resilience, and channel partnerships for last-mile delivery. Leaders are investing in extensive clinical evidence generation to demonstrate long-term safety, real-world effectiveness, and comparative advantages against oral regimens. This evidence underpins value propositions to payers and supports inclusion in guidelines and procurement tenders.Manufacturing scale and supply-chain resilience are strategic differentiators. Organizations that secure diversified raw material sources, expand contract manufacturing networks, or develop domestic production capabilities are reducing exposure to trade volatility and tariffs. Concurrently, companies are forming partnerships with specialty clinics, community health organizations, and logistics providers to build reliable distribution pathways, including direct-to-patient mechanisms that preserve continuity and improve adherence.
Commercially, firms are designing flexible pricing models and pilot reimbursement arrangements that reflect different health-system realities. Collaboration with non-traditional stakeholders-such as community-based organizations, telehealth platforms, and public procurement agencies-enables tailored rollouts and helps build demand. Taken together, these strategic moves show a shift from product-centric to systems-aware commercialization, where successful entrants will combine robust clinical data with adaptive supply and distribution capabilities.
Practical strategic moves for executives to secure supply resilience, evidentiary differentiation, and scalable distribution that accelerate equitable uptake
Industry leaders should prioritize integrated strategies that align clinical, commercial, and operational functions to accelerate sustainable adoption. First, invest in molecule- and device-specific evidence generation that addresses long-term safety, interaction profiles, and real-world adherence outcomes; these data are essential for payer negotiations and guideline inclusion. Second, build supply-chain resilience by diversifying raw material sources, evaluating nearshoring options, and negotiating flexible contracting terms to buffer against tariff-related disruptions and component shortages.Next, expand distribution portfolios to include direct-to-patient mechanisms and strengthen ties with community health centers and specialty clinics to reach diverse populations. Embed patient-centric services such as telehealth follow-up, mobile procedural units, and adherence support tools to reduce friction and improve retention. Align pricing and reimbursement strategies with local payer expectations through outcome-based pilot programs and multi-stakeholder value dialogues.
Finally, coordinate with regulators and public health agencies early in the introduction process to streamline approvals, harmonize safety monitoring, and design scalable training programs for providers. These measures will reduce rollout friction, protect margins against short-term cost pressures, and ensure that access considerations remain central to commercial planning.
A transparent mixed-methods approach integrating stakeholder interviews, clinical literature review, regulatory analysis, and scenario modeling to ensure robust and actionable insights
The research underpinning this analysis combined qualitative and quantitative approaches to produce a robust, triangulated understanding of the parenteral PrEP landscape. Primary research included structured interviews with clinicians, procurement specialists, payers, device and pharmaceutical development leads, and community health program managers to capture operational realities, evidence needs, and distribution preferences. These interviews were complemented by reviews of peer-reviewed literature, clinical trial registries, regulatory guidance documents, and technical standards for implantable devices and injectable therapies.Secondary sources informed contextual understanding of supply-chain dynamics, polymer and component sourcing, and logistics considerations, while scenario analysis explored the implications of tariff shocks and regional rollout strategies. Data synthesis involved cross-validation across sources to reconcile differences in viewpoints and to highlight areas of consensus and uncertainty. The methodology prioritized transparency in assumptions, and findings emphasize actionable implications rather than speculative forecasting, ensuring relevance for decision-makers planning introductions and scale-up.
Integrated conclusions that clarify why coordinated clinical evidence, supply strategies, and delivery innovations are essential to realize the full prevention potential of parenteral PrEP
Parenteral PrEP represents a pivotal evolution in HIV prevention, offering new ways to deliver durable protection that align with patient preferences and health-system constraints. Success will hinge on coordinated strategies that marry strong clinical evidence with resilient supply chains, adaptive distribution models, and payer-aligned value propositions. As the field matures, stakeholders who proactively address device lifecycle management, molecule-specific considerations, and regional infrastructure differences will accelerate access and maximize public health impact.The interplay of tariffs, regional regulatory environments, and heterogeneous end-user needs underscores the importance of flexible planning and multi-stakeholder collaboration. Whether through partnerships that extend last-mile reach, investments in domestic production capacity, or innovative reimbursement pilots, the ability to translate product attributes into operational readiness will determine which interventions achieve sustained uptake. This conclusion emphasizes the imperative for integrated action across clinical development, manufacturing, and service delivery to realize the full potential of parenteral PrEP.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- Accord Healthcare Inc.
- Amneal Pharmaceuticals, Inc.
- Aurobindo Pharma Ltd.
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Fresenius Kabi AG
- Gilead Sciences, Inc.
- Hetero Labs Limited
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- ViiV Healthcare Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
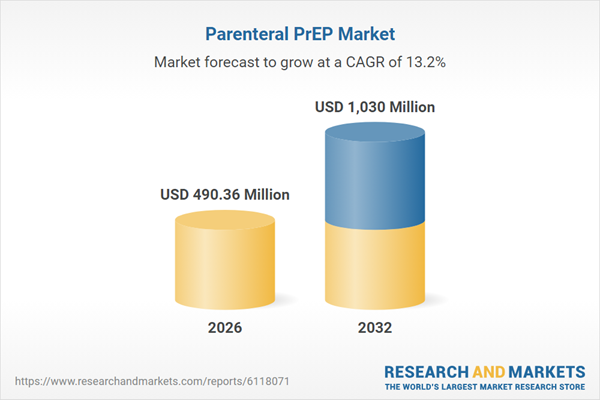
| Estimated Market Value ( USD | $ 490.36 Million |
| Forecasted Market Value ( USD | $ 1030 Million |
| Compound Annual Growth Rate | 13.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |