Speak directly to the analyst to clarify any post sales queries you may have.
A clear and pragmatic orientation to gene-based therapeutic advances redefining clinical pathways and stakeholder responsibilities in sickle cell disease
Sickle cell disease stands at a critical juncture where decades of pathobiological research intersect with advanced molecular interventions capable of altering disease trajectory. The introduction of gene-modifying approaches has reframed clinical goals from symptomatic management toward durable modification of the underlying hematologic defect. As a result, clinicians, payers, and health systems are recalibrating clinical pathways to integrate curative-intent therapies alongside established supportive care.This introduction presents a concise orientation to the field, highlighting how novel modalities have reshaped therapeutic intent, trial design, and patient selection. It situates gene therapies within a continuum of care that encompasses pre-treatment conditioning, peri-procedural management, and longitudinal follow-up for safety and efficacy assessment. Importantly, this section clarifies the practical implications for stakeholders who must balance clinical promise with operational complexity, such as infusion logistics, specialized center accreditation, and post-therapy monitoring protocols.
By framing the conversation around translational milestones and real-world readiness, the introduction prepares readers to engage with subsequent sections that unpack systemic shifts, regulatory influences, payer dynamics, and actionable recommendations. Throughout, the emphasis is on pragmatic interpretation of scientific advances so that decision-makers can prioritize investments, clinical partnerships, and policy dialogues that support responsible adoption and equitable access.
How scientific maturation, regulatory adaptation, and integrated care investments are reshaping adoption pathways and long-term management for gene therapies in sickle cell disease
The therapeutic landscape for sickle cell disease is undergoing transformative shifts driven by scientific maturation, regulatory evolution, and evolving care infrastructure. Recent years have seen gene-modifying techniques progress from conceptual frameworks to human studies that demonstrate durable effects on hemoglobin expression and clinical endpoints. Consequently, clinical trial design has become more sophisticated, prioritizing long-term safety surveillance and functional outcomes that matter to patients, such as decreased vaso-occlusive episodes and organ protection.Regulatory agencies are adapting pathways to accommodate these complex therapies, implementing frameworks for accelerated assessment while emphasizing robust post-approval commitments. At the same time, health systems are investing in specialized centers and interdisciplinary teams to manage peri-transplant conditioning, immune suppression, and long-term monitoring, recognizing that successful deployment requires more than a single procedural episode.
Moreover, payers and policy actors are exploring innovative payment models to address the high upfront costs and to align reimbursement with long-term value realization. Stakeholder collaboration has intensified, with academic consortia, patient advocacy organizations, and industry partners co-developing registries and standardized outcome measures. Taken together, these shifts signal a move from isolated experimental interventions toward integrated care pathways that support both clinical effectiveness and systems-level sustainability.
Assessing the downstream operational and procurement effects of tariff shifts on supply chains, manufacturing localization, and affordability for advanced sickle cell therapies
Policy decisions and tariff regimes can materially affect the deployment of advanced therapies, and recent trade adjustments have prompted industry stakeholders to re-evaluate cross-border supply strategies and pricing structures. Tariff changes influence the cost base for imported biologics, vector components, and specialized consumables required for gene-modifying procedures, thereby affecting procurement planning for hospitals and specialty treatment centers.In response to shifting tariff landscapes, manufacturers and supply chain partners are implementing mitigation strategies that include localized manufacturing partnerships, dual-sourcing of critical components, and supply chain segmentation to prioritize continuity of care. These adaptations reduce exposure to single-point disruptions and create flexibility in logistics, but they also necessitate additional capital and technical oversight to ensure manufacturing comparability and regulatory compliance across jurisdictions.
From a payer perspective, tariff-driven cost fluctuations amplify the importance of contract design that accommodates variable input costs and secures predictable access for patient populations. Health systems must therefore integrate tariff risk into procurement cycles, contracting timelines, and budget impact assessments, while also engaging with policymakers to communicate the downstream clinical and economic consequences of trade policy on access to transformative treatments.
A comprehensive segmentation framework linking treatment model, genetic modification technology, patient demographics, disease severity, payer type, and care delivery settings to strategic decision-making
Understanding market dynamics requires a nuanced segmentation approach that reflects treatment modality, genetic manipulation strategy, patient demographics, clinical severity, payer structure, and care setting. Differentiation by treatment model distinguishes therapies based on whether they employ donor-derived allogeneic approaches or patient-specific autologous methods, with each pathway presenting distinct logistics, immunologic considerations, and conditioning regimens. Meanwhile, advances in modification technology delineate therapeutic candidates by precision and mechanism; base editing and CRISPR/Cas9 methods offer highly targeted genomic alteration, lentiviral vector strategies enable gene addition or correction through stable integration, and zinc finger nucleases provide an alternative programmable nuclease platform, each with unique profiles for off-target risk and manufacturing requirements.Patient age segmentation highlights divergent clinical priorities and operational needs across adult and pediatric populations, as younger patients may derive long-term benefits from early intervention while older cohorts present distinct comorbidity and consent considerations. Severity stratification between moderate and severe disease states shapes candidate selection and expected benefit-risk tradeoffs, influencing both clinical decision-making and ethical frameworks for treatment allocation. Payer category distinctions among government programs, out-of-pocket financing, and private insurance create heterogeneous reimbursement pathways and coverage criteria, necessitating tailored value demonstration and engagement strategies. Finally, distribution channel segmentation underscores where therapies will be delivered: hospitals provide integrated inpatient capabilities for conditioning and complex supportive care, whereas specialty treatment centers offer concentrated expertise and potentially streamlined patient pathways for cell processing and follow-up care. Taken together, these segmentation lenses allow stakeholders to align product development, clinical operations, and commercial approaches with the specific needs and constraints of each patient and system context.
How regional differences in clinical capacity, regulatory frameworks, and payer environments drive differentiated adoption and access strategies for gene therapies across global regions
Regional dynamics exert a powerful influence on how gene therapies for sickle cell disease are researched, regulated, and adopted, with each geographic area exhibiting distinct clinical infrastructure, policy priorities, and patient needs. In the Americas, concentrated centers of scientific innovation and established transplant programs provide fertile ground for clinical trials and early adoption, while policy debates around reimbursement and equitable access drive collaborative pilot programs that seek to broaden reach beyond major academic centers. Regulatory agencies in this region have been refining accelerated pathways that still require comprehensive long-term safety commitments, which shapes post-approval evidence generation and surveillance strategies.Across Europe, Middle East & Africa, heterogeneous health systems and variable infrastructure present both challenges and opportunities. High-resource European countries often focus on integrating advanced therapies into national health frameworks with strong emphasis on health technology assessment and long-term outcome data, whereas parts of the Middle East and Africa face constraints in specialized care capacity and supply chain readiness that necessitate alternative models of care delivery and capacity-building initiatives.
In the Asia-Pacific region, a combination of rapidly expanding biotech ecosystems, large patient populations, and increasing regulatory harmonization creates potential for accelerated clinical development and regional manufacturing scale. However, diverse regulatory requirements and reimbursement landscapes mean that market entry strategies must be adaptable and region-specific. Overall, regional strategies should be informed by local clinical capacity, regulatory expectations, payer mechanics, and patient advocacy dynamics to ensure responsible and equitable deployment of gene-based interventions.
How technology platforms, manufacturing capabilities, and strategic partnerships among leading developers and clinical consortia are shaping therapeutic pathways and operational readiness
Leading biomedical organizations and clinical consortia are advancing a spectrum of gene-based approaches targeting the molecular drivers of sickle cell disease, and their activities shape competitive dynamics as well as collaborative opportunities. Industry participants range from translational biotechnology companies developing novel editing platforms to established biologics manufacturers scaling vector production and distribution networks. Academic centers and cooperative research groups contribute foundational clinical evidence and long-term outcome data, often partnering with commercial developers to conduct pivotal studies and to establish real-world registries.Key players are distinguishing themselves through proprietary platform technologies, manufacturing capacity for viral and non-viral delivery systems, and strategic alliances that extend clinical reach into community and specialty settings. Investment in manufacturing standardization, quality systems, and comparator studies is creating differentiated capability to meet regulatory expectations for product consistency and safety. At the same time, a growing ecosystem of supportive service providers-ranging from cell processing laboratories to data management vendors-enables end-to-end solutions that lower operational barriers for treating centers. Collectively, these company-level moves are accelerating pathway maturation while also highlighting the importance of partnerships that align scientific innovation with pragmatic considerations for scale and access.
Actionable strategic priorities for industry leaders to align clinical innovation, manufacturing scale, reimbursement design, and equitable access in advancing gene therapies
Industry leaders must take deliberate steps to bridge scientific promise and system-level delivery to ensure sustainable access to gene therapies for sickle cell disease. First, invest in integrated care networks that combine hospital infrastructure with specialty treatment centers and community outreach to streamline patient identification, pre-treatment preparation, and longitudinal monitoring. Second, develop adaptive reimbursement approaches that share risk and reward across stakeholders, such as outcomes-based contracts and multi-year payment arrangements, to mitigate upfront cost barriers while aligning incentives around long-term clinical benefit.Third, prioritize scalable manufacturing strategies that include regional production partnerships, technology transfer protocols, and rigorous comparability studies to maintain quality across sites. Fourth, engage proactively with regulators and payers to define meaningful endpoints, acceptable evidence thresholds for approval and coverage, and post-market surveillance mechanisms that balance timely access with safety oversight. Fifth, commit resources to equitable access initiatives, including capacity-building in under-resourced regions and support programs that address socioeconomic barriers to treatment. By executing these interconnected actions, industry leaders can convert clinical innovation into durable improvements in patient outcomes while maintaining financial and operational viability.
A transparent, reproducible research approach combining scientific literature, regulatory guidance, clinical practice input, and stakeholder interviews to inform implementation-focused insights
This research synthesizes evidence from peer-reviewed literature, regulatory guidances, clinical trial registries, and stakeholder interviews to construct an integrated view of the gene therapy landscape for sickle cell disease. The analysis triangulates mechanistic insights from basic science with operational realities reported by treating centers and manufacturers, placing emphasis on real-world readiness, regulatory expectations, and payer engagement strategies. Where appropriate, methodological transparency is maintained by documenting inclusion criteria for source material, timeframes for literature coverage, and the composition of expert interview panels.Qualitative inputs were systematically coded to identify recurring themes related to clinical outcomes, safety considerations, manufacturing constraints, and access barriers. Comparative technology assessment focused on mechanistic differentiation, manufacturing complexity, and known safety profiles rather than speculative performance claims. Regional analysis incorporated regulatory publications and policy statements, while stakeholder perspectives helped contextualize implementation challenges at the provider and payer levels. Throughout, analytic judgments prioritize reproducibility and clarity, and limitations are acknowledged regarding the evolving nature of the field and ongoing data generation that will refine current understanding.
A forward-looking synthesis emphasizing coordinated investments in manufacturing, equitable access, and evidence generation to realize clinical and system-level benefits from gene therapies
The trajectory of gene therapy for sickle cell disease points to substantive changes in clinical practice and health system organization, contingent upon coordinated progress across science, manufacturing, policy, and clinical operations. Early clinical successes have validated the underlying biological hypotheses and have triggered essential conversations about how to scale complex interventions responsibly. Yet, translating these innovations into broad-based patient impact requires deliberate alignment between technical capabilities and system incentives, including investments in specialized care delivery, adaptive reimbursement, and long-term safety monitoring.As the landscape evolves, stakeholders who proactively address manufacturing robustness, equitable access, and evidence generation will be best positioned to lead adoption and to demonstrate value. Collaboration across the public and private sectors-grounded in transparent data sharing and patient-centered outcome measures-will accelerate safe and ethical deployment. Ultimately, careful stewardship of scientific advances, paired with pragmatic operational planning, can realize the potential of gene-based interventions to transform care for people living with sickle cell disease.
Table of Contents
19. ResearchStatistics
20. ResearchContacts
21. ResearchArticles
22. Appendix
Companies Mentioned
- Agios Pharmaceuticals, Inc.
- Beam Therapeutics, Inc.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- CRISPR Therapeutics AG
- Editas Medicine, Inc.
- EditForce, Inc.
- Emmaus Life Sciences, Inc.
- F. Hoffmann-La Roche Ltd.
- Fulcrum Therapeutics, Inc.
- Global Blood Therapeutics, Inc.
- Graphite Bio, Inc.
- Homology Medicines, Inc.
- Imara, Inc.
- Incyte Corporation
- Intellia Therapeutics, Inc.
- JCR Pharmaceuticals Co., Ltd.
- Magenta Therapeutics, Inc.
- Novartis AG
- Pfizer Inc.
- Precision BioSciences, Inc.
- Sangamo Therapeutics, Inc.
- Sanofi S.A.
- Vertex Pharmaceuticals Incorporated
- Vifor Pharma AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
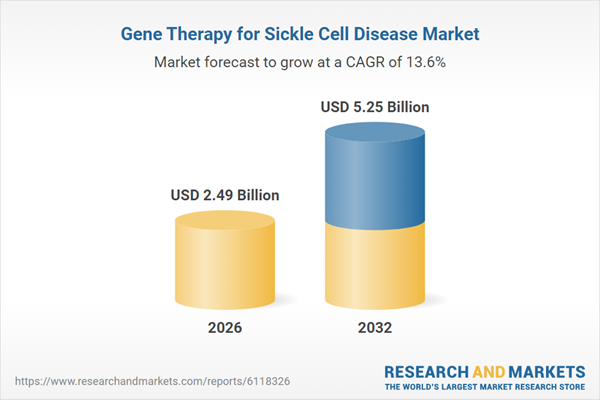
| Estimated Market Value ( USD | $ 2.49 Billion |
| Forecasted Market Value ( USD | $ 5.25 Billion |
| Compound Annual Growth Rate | 13.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |