Speak directly to the analyst to clarify any post sales queries you may have.
Contextualizing EGFR testing as an indispensable precision oncology tool shaping diagnostics workflows clinical decision-making and multidisciplinary care pathways
Epidermal growth factor receptor (EGFR) testing has shifted from a specialized niche into a cornerstone of precision oncology diagnostics, underpinning treatment selection, clinical trial enrollment, and post-therapy monitoring across multiple tumor types. In clinical practice, EGFR status informs targeted therapy decisions and increasingly serves as a dynamic biomarker when assessed longitudinally through minimally invasive sampling. As a result, laboratories and clinical programs must reconcile legacy workflows with an expanding suite of assay modalities, while healthcare systems adapt pathways to incorporate timely molecular results.Throughout care pathways, the demand for higher analytical sensitivity, shorter turnaround times, and integrated reporting has redefined expectations for assay performance and laboratory interoperability. Consequently, multidisciplinary teams-from oncologists to laboratory scientists and payers-are recalibrating procedural protocols and resource allocation to ensure that molecular diagnostics deliver clinically actionable results when they matter most.
In this context, testing technology, regulatory clarity, and reimbursement frameworks converge to shape adoption rates and operational models. As stakeholders navigate these interdependencies, they must prioritize scalability, quality management, and clinical validation. This introduction sets the stage for a deeper examination of the technological, regulatory, and commercial forces driving evolution in EGFR testing strategies and service delivery.
Technological convergence and clinical evidence advancement reshaping EGFR diagnostics with implications for testing pathways regulatory clarity and payer alignment
The landscape for EGFR diagnostics is undergoing transformative shifts driven by technological innovation, evolving clinical evidence, and the maturation of value-based care models. Next-generation sequencing has expanded the diagnostic horizon by enabling concurrent multi-gene interrogation, thereby transitioning many testing algorithms from single-gene assays to comprehensive panels that provide richer biological context for therapeutic selection and clinical trial matching.Concurrently, liquid biopsy platforms are increasingly capable of detecting low-frequency variants and monitoring clonal dynamics over time, offering clinicians a practical route to longitudinal disease surveillance without repeated invasive procedures. This shift is complemented by advances in bioinformatics and machine learning, which enhance variant interpretation, reduce false positives, and streamline report generation for busy clinical teams. In parallel, improvements in PCR-based assays and digital PCR technologies maintain an important role where rapid, cost-effective, and highly sensitive detection of specific EGFR alterations is required for immediate treatment decisions.
Regulatory and payer environments are also evolving; regulatory clarity around companion diagnostics and reimbursement frameworks that recognize diagnostic-driven therapeutic value are incentivizing assay standardization and clinical validation. As institutions adapt to these changes, partnerships between diagnostic developers, clinical laboratories, and pharmaceutical companies are becoming more strategic, with an emphasis on co-development, real-world evidence generation, and integrated clinical pathways that deliver measurable patient benefit.
Assessing how changes in import tariff policy affect diagnostic supply chains procurement strategies and clinical continuity for EGFR testing services
Policy shifts in tariff regimes can reverberate across laboratory supply chains and capital procurement choices, with cumulative effects on test availability, cost structures, and supplier portfolios. When tariffs are applied to imported reagents, sequencing consumables, and instrumentation components, diagnostic providers face immediate procurement cost pressures that may affect purchasing cadence, inventory strategies, and long-term supplier relationships. These impacts are further amplified in systems where supply chains are already constrained or where single-source suppliers dominate critical reagent lines.In response to tariff-induced cost pressures, many clinical laboratories and diagnostic companies pursue supply diversification, including regional sourcing, strategic stockpiling, and increased use of alternative assay chemistries that rely less on tariff-exposed components. Simultaneously, instrument vendors have begun to re-evaluate manufacturing footprints and consider nearshoring to mitigate exposure to cross-border levies. Such operational shifts can produce short-term disruptions but may also catalyze longer-term resilience through localized production and strengthened vendor redundancy.
For clinicians and patients, the most tangible consequences can include extended lead times for instrument installation or reagent restocking, selective prioritization of tests, and, in some cases, higher per-test operational costs. Over time, sustained tariff pressure can incentivize domestic capacity building and spur innovation in reagent formulations and instrument design that are less supply-chain dependent. Ultimately, the ability of diagnostic stakeholders to adapt operationally and contractually will determine how tariff dynamics translate into clinical continuity and access to EGFR testing.
Clarifying the clinical and operational implications of assay modalities specimen types indications and end-user priorities to guide strategic segmentation and service design
Segmentation analysis reveals where clinical utility, operational feasibility, and commercial focus converge across assay formats, specimen modalities, clinical indications, and end-user types. Based on Test Type, the market is studied across Immunohistochemistry (IHC) Tests, In Situ Hybridization (ISH), Next-Generation Sequencing (NGS) Panels, and PCR-based Assays, each of which carries distinct performance characteristics and workflow implications that influence where they are most effectively deployed in care pathways. IHC and ISH retain value for protein expression and gene-level assessments within histopathology workflows, whereas NGS panels provide comprehensive molecular profiling and PCR-based assays deliver rapid, targeted mutation detection.Based on Sample Type, the market is studied across Liquid Biopsy and Tissue Biopsy, with liquid biopsy enabling serial monitoring and minimally invasive genotyping while tissue biopsy remains the diagnostic standard for initial histologic and staging assessments. Clinical application is further refined by the range of indications, and based on Application, the market is studied across Breast Cancer, Glioblastomas, Head and Neck Cancers, and Non-Small Cell Lung Cancer (NSCLC), where differing prevalence of actionable EGFR alterations and clinical management paradigms necessitate tailored testing algorithms.
Finally, laboratory and commercial strategy are informed by the distribution of end-users, and based on End-Users, the market is studied across Academic & Research Institutions, Diagnostic Laboratories, Hospitals & Cancer Centers, and Pharmaceutical & Biotechnology Companies. Each end-user group balances priorities differently-academic centers prioritize innovation and depth of data, diagnostic laboratories emphasize throughput and cost-efficiency, hospitals focus on clinical integration and turnaround, and pharmaceutical partners require companion diagnostic alignment and trial-ready assays.
Examining how regional healthcare infrastructure regulatory convergence and local manufacturing capacity influence EGFR test access adoption and partnership strategies
Regional dynamics shape access models, regulatory pathways, and partnership strategies for EGFR testing, reflecting differences in healthcare infrastructure, clinical adoption, and local manufacturing capacity. Across the Americas, diagnostic networks vary from highly centralized reference laboratories to decentralized hospital-based testing, and this diversity influences how quickly novel assay formats are adopted and scaled into routine practice. In many parts of the Americas, strong connections between clinical trial networks and diagnostic developers accelerate translational uptake, yet reimbursement variability continues to shape test placement and laboratory investment cycles.In Europe, Middle East & Africa, regulatory harmonization initiatives and pan-regional collaborations are creating pathways for more consistent test validation and cross-border clinical studies, but disparities in healthcare funding and laboratory infrastructure require adaptable service models. Capacity building and targeted training in molecular pathology remain critical to unlock broader access to advanced EGFR testing across diverse healthcare settings within this region.
Across Asia-Pacific, rapid adoption of molecular diagnostics is supported by significant investments in biotechnology and diagnostic manufacturing, with some markets demonstrating strong domestic supply chains for reagents and instruments. This region often leads in deployment scale for high-throughput sequencing and population-level screening initiatives, yet the regulatory landscape and reimbursement timelines differ markedly between countries, which influences commercialization strategies and partnership models. Across regions, tailored approaches to regulatory engagement, supply chain resilience, and clinical education are essential to ensure consistent access to high-quality EGFR diagnostics.
Understanding competitive positioning through assay specialization strategic partnerships and integrated analytics that drive adoption and clinical utility in EGFR diagnostics
Competitive dynamics in EGFR diagnostics are characterized by a mix of established instrument manufacturers, specialist assay developers, clinical laboratories, and emerging digital pathology and bioinformatics providers. Incumbent companies continue to leverage broad installed bases and integrated reagent ecosystems to maintain market presence, while nimble specialist firms focus on high-sensitivity assays, liquid biopsy innovation, and streamlined reporting solutions that meet specific clinical needs. These dual strategies drive a stratified competitive environment in which scale and specialization coexist.Strategic collaborations between diagnostic developers and pharmaceutical companies remain a core pathway for bringing companion diagnostics to market, with co-development agreements increasingly emphasizing shared data generation and regulatory alignment. Similarly, mergers and alliances among clinical laboratory networks and technology providers have accelerated the consolidation of testing services, enabling optimized logistics and standardized quality assurance across geographies. At the same time, software and analytics companies are becoming indispensable partners by offering interpretation layers that translate raw molecular data into clinically actionable reports.
Taken together, these trends suggest that sustained competitive advantage will come from integrated offerings that combine robust assay performance, validated clinical utility, scalable operations, and comprehensive data interpretation. Organizations that invest in interoperable platforms and cultivate long-term partnerships across clinical, commercial, and regulatory stakeholders will be best positioned to influence adoption trajectories for EGFR testing.
Operational and strategic levers for diagnostic leaders to build resilient assay portfolios strengthen payer engagement and accelerate clinical adoption of EGFR testing
Industry leaders should adopt a proactive, multi-dimensional strategy to capitalize on clinical demand for accurate and timely EGFR diagnostics while managing operational risk and payer expectations. First, prioritize investments in assay portfolios that balance immediate clinical needs with longer-term strategic value: maintain rapid PCR-based pathways for urgent treatment decisions while expanding validated NGS offerings and building liquid biopsy capabilities for longitudinal monitoring. Parallel investments in robust bioinformatics and standardized reporting templates will reduce interpretation variability and enhance clinician confidence.Second, strengthen supply chain resilience by diversifying reagent sources, negotiating longer-term supplier agreements, and exploring regional manufacturing partnerships to mitigate exposure to trade policy volatility. Concurrently, engage early with regulatory authorities and payers to align evidence generation with reimbursement requirements, and collaborate with pharmaceutical partners to design companion diagnostic studies that produce clinically actionable endpoints. Attention to operational excellence-through quality management systems, laboratory accreditation, and automation-will support predictable turnaround times and cost control.
Finally, cultivate clinical adoption through targeted education programs, real-world evidence initiatives, and multidisciplinary pathway integration that demonstrate tangible patient benefits. By combining technical excellence, operational resilience, and focused stakeholder engagement, organizations can accelerate sustainable adoption of EGFR testing while preserving flexibility in the face of evolving clinical and policy environments.
A multi-method evidence synthesis combining stakeholder interviews technical validation review and competitive mapping to inform strategic decisions in EGFR diagnostics
This research synthesis integrates a multi-method approach designed to triangulate technical, clinical, and commercial perspectives. Primary qualitative inputs included structured interviews with laboratory directors, molecular pathologists, hospital administrators, and diagnostic product leaders to capture operational constraints, validation experiences, and adoption drivers. These stakeholder insights were complemented by systematic reviews of clinical guidelines, peer-reviewed literature, and regulatory communications to ensure alignment with contemporary standards of care and diagnostic validation requirements.Technical evaluation assessed assay performance attributes-analytical sensitivity, specificity, sample requirements, and workflow compatibility-through comparison of published validation studies and publicly available technical specifications. Competitive analysis synthesized company disclosures, product portfolios, and partnership announcements to map strategic positioning and innovation trajectories. Where appropriate, supply chain resilience was examined through procurement practice reviews and supplier ecosystem mapping to identify critical dependencies and mitigation strategies.
Throughout the research process, findings were validated via cross-checked sources and follow-up discussions with subject matter experts to ensure interpretive accuracy. Limitations include variability in public disclosure of proprietary validation data and the dynamic nature of regulatory processes, which may evolve after data collection. Nevertheless, the methodology provides a robust, multi-perspective foundation for strategic decision-making in EGFR diagnostics.
Synthesizing technological promise operational realities and stakeholder alignment to outline strategic imperatives for effective EGFR testing deployment
EGFR testing sits at the intersection of advancing molecular technologies, evolving clinical imperatives, and complex operational realities. The convergence of comprehensive sequencing, accessible liquid biopsy options, and refined bioinformatics creates unprecedented opportunities to deliver personalized therapeutic strategies across multiple tumor types. Yet realizing this potential requires deliberate alignment of assay selection, laboratory capabilities, and stakeholder engagement to ensure that diagnostic insight reliably translates into clinical action.Operational resilience and supply chain diversification are now strategic imperatives, particularly in the face of external pressures that can affect reagent availability and instrument procurement. At the same time, payer engagement and regulatory alignment remain critical enablers of broader clinical adoption. Institutions that invest in validated, interoperable workflows and partner effectively with clinical and commercial stakeholders will be best positioned to deliver high-quality EGFR diagnostics at scale.
In conclusion, the path forward demands integrated strategies that combine technical excellence, collaborative partnerships, and adaptive operations. By doing so, healthcare organizations and diagnostic innovators can enhance patient access to targeted therapies and embed EGFR testing as a reliable element of precision oncology care.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China EGFR Tests Market
Companies Mentioned
- 3B BlackBio Dx Limited
- Abbexa Ltd.
- Abbott Laboratories
- Agilent Technologies, Inc.
- AstraZeneca plc
- Beijing ACCB Biotech Ltd.
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Biomatik Corporation
- F. Hoffmann-La Roche AG
- Foundation Medicine, Inc.
- Guardant Health, Inc.
- Illumina, Inc.
- Luminex Corporation
- MyBiosource, Inc.
- Myriad Genetics, Inc.
- QIAGEN N.V.
- RainSure Scientific
- Shanghai Korain Biotech Co., Ltd.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
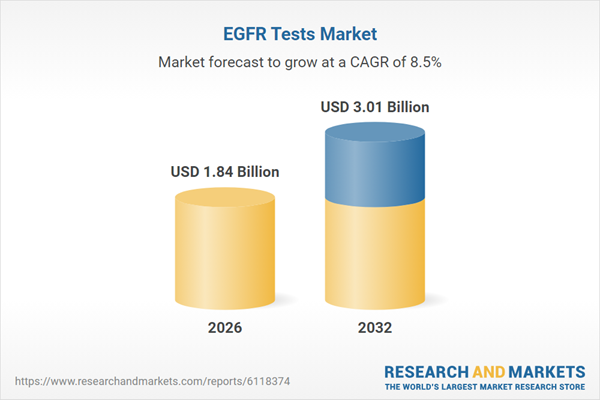
| Estimated Market Value ( USD | $ 1.84 Billion |
| Forecasted Market Value ( USD | $ 3.01 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |