Speak directly to the analyst to clarify any post sales queries you may have.
A clear-sighted introduction to the evolving clinical rationale, care delivery shifts, and strategic priorities shaping oral therapies for Gaucher disease
Gaucher's disease represents a complex, genetically driven lysosomal storage disorder that has prompted sustained interest in therapeutic innovation and patient-centric care models. This introduction frames the clinical, commercial, and operational considerations that matter most to stakeholders engaged in advancing oral therapy solutions for Gaucher's disease. It outlines the medical rationale driving interest in substrate reduction strategies alongside established enzyme replacement approaches and emerging gene-based modalities, and it clarifies why oral therapies occupy a unique position in treatment paradigms due to convenience, adherence potential, and outpatient delivery implications.Clinicians and program leaders must account for heterogeneity in disease presentation, spanning neuronopathic and non-neuronopathic forms, and the variable care needs of adult and pediatric populations. Meanwhile, payers and distributors face evolving criteria for reimbursement and access as oral formulations alter the traditional hospital-centric infusion model. Consequently, strategic decisions around clinical development, evidence generation, and market access require integrated thinking that balances efficacy, safety, long-term outcomes, and real-world utility.
As we proceed, the reader should expect rigorous synthesis of therapeutic mechanisms, regulatory trends, stakeholder expectations, and practical challenges that influence adoption. This introduction sets the stage for an executive-level conversation about how oral therapies for Gaucher's disease may reshape care delivery, engage new distribution channels, and catalyze shifts in research priorities across clinical and commercial domains.
How diagnostic advances, regulatory evolution, and patient-centered expectations are accelerating therapeutic innovations and reshaping care pathways for Gaucher disease
Recent years have produced transformative shifts that are redefining therapeutic development and care pathways for rare metabolic diseases, with direct implications for oral therapy approaches in Gaucher's disease. Advances in molecular diagnostics and next-generation sequencing have expedited diagnosis and genotype-phenotype correlations, enabling more precise patient stratification and targeted trial enrollment. Simultaneously, improvements in drug delivery science have increased the feasibility of orally bioavailable agents that achieve meaningful target engagement, prompting a reevaluation of long-standing reliance on parenteral enzyme replacement regimens.Regulatory frameworks have also adapted to the realities of rare disease drug development, offering more flexible pathways for accelerated review and conditional approvals when robust surrogate endpoints or compelling natural history data exist. In parallel, stakeholder expectations have shifted: patients and caregivers increasingly prioritize quality-of-life outcomes and convenience, while payers seek durable evidence of cost-effectiveness and adherence benefits. This confluence of scientific innovation, regulatory adaptation, and patient-centered demand has moved the landscape toward therapies that not only address biochemical deficits but also fit within everyday life.
Operationally, manufacturers and clinical teams are experimenting with decentralized trial models and digital monitoring to capture long-term outcomes outside traditional clinic settings. These approaches reduce patient burden and generate real-world evidence that can complement clinical trial data, supporting broader acceptance of oral therapies. Taken together, these shifts create a fertile environment for strategic investment, but they also require careful orchestration among clinical research, regulatory affairs, and commercial planning functions to translate promise into sustainable patient access.
Assessing how trade tariff shifts in 2025 have pressured supply chains, manufacturing choices, and commercial strategies with implications for patient access and cost management
Policy changes in trade and tariff regimes can ripple across pharmaceutical supply chains and commercial strategies, and the cumulative impact of tariffs enacted in 2025 in the United States has generated specific operational and strategic consequences for manufacturers and distributors involved in rare disease therapies. Tariff-related cost increases on imported active pharmaceutical ingredients, specialized excipients, and certain manufacturing equipment have pressured margins, prompting some companies to reassess supplier relationships and to consider dual sourcing or regional manufacturing alternatives. As a result, decision-making now increasingly factors in logistics resilience alongside clinical development priorities.These trade dynamics have also influenced procurement planning for distributors and hospital supply chains, with inventory management practices shifting to favor longer lead times and safety stock for critical components. For therapies that transition from hospital-based infusions to oral administration, the cost calculus of distribution and storage changes, but import duties and customs costs still affect the landed price and, by extension, discussions with payers and reimbursement authorities. Consequently, manufacturers are undertaking scenario planning that models cost-to-serve under different tariff regimes and explores contractual protections with suppliers.
In response, organizations have accelerated efforts to localize aspects of the value chain, including formulation and secondary packaging, where feasible. These moves aim both to reduce exposure to external tariff volatility and to shorten time-to-market in key jurisdictions. At the same time, stakeholders are engaging earlier with policymakers and industry groups to clarify tariff classifications for pharmaceutical goods and to seek exemptions or mitigations for critical medical supplies. Overall, the tariff environment has underscored the importance of supply chain agility, cross-functional risk management, and proactive engagement with trade authorities to preserve patient access and commercial viability.
Segment-driven strategic insights that link therapeutic modalities, patient subgroups, distribution channels, and care settings to practical development and access priorities
A nuanced view of segmentation illuminates where therapeutic opportunity and clinical need intersect across therapy class, disease phenotype, patient demographics, distribution pathways, and end-user profiles. Based on therapy type, the market encompasses Enzyme Replacement Therapy, Gene Therapy, and Substrate Reduction Therapy, where the enzyme replacement category further divides into specific biologic agents such as Imiglucerase, Taliglucerase, and Velaglucerase. Gene therapy approaches include both AAV based strategies and lentiviral platforms, with the AAV based subset differentiated into serotype-specific vectors including AAV2 and AAV9. Substrate reduction therapy is represented by oral agents such as Eliglustat and Miglustat, each carrying distinct pharmacologic profiles that influence tolerability and monitoring requirements.Disease type segmentation spans Type 1, Type 2, and Type 3 presentations, reflecting differences in visceral, hematologic, and neurologic involvement that direct therapeutic selection and outcome measurement. Patient age group segmentation distinguishes Adult and Pediatric populations, necessitating divergent safety assessments, dosing strategies, and caregiver considerations. Distribution channel segmentation differentiates Hospital Pharmacies and Retail Pharmacies, with hospital pharmacies further classified into private and public institutions that vary in procurement processes and formularies. End user segmentation covers Clinics and Hospitals, and hospitals themselves are viewed through the lens of general hospitals versus specialty facilities where rare disease expertise and multidisciplinary teams are more concentrated.
Understanding these intersecting segments enables stakeholders to target evidence generation and commercial initiatives more effectively. For example, oral substrate reduction therapies may be prioritized for adult, non-neuronopathic populations with established outpatient follow-up, whereas gene therapy programs often concentrate on younger patients with severe phenotypes and require specialized center-of-excellence delivery models. Distribution strategies likewise adjust based on whether hospital-based specialty pharmacies or community retail networks will better support adherence and long-term monitoring. By integrating segmentation insights into program design, sponsors and providers can align clinical development with realistic pathways to patient access.
Regional regulatory, reimbursement, and healthcare delivery differences that determine adoption pathways and access strategies across major global markets
Regional dynamics play a central role in shaping how therapies for Gaucher disease progress from development to clinical use, and a focused regional analysis clarifies regulatory expectations, reimbursement pathways, and stakeholder preferences. In the Americas, regulatory authorities and payer frameworks tend to emphasize comparative effectiveness and health economics evidence, while patient advocacy organizations play a strong role in driving diagnosis awareness and early intervention. This region often features a mix of centralized specialty centers and community-based clinics that together determine uptake patterns for oral versus parenteral therapies.Across Europe, Middle East & Africa, regulatory landscapes are diverse, with some countries offering expedited pathways for rare disease treatments and others applying more stringent health technology assessment standards that judge long-term value. Publicly funded healthcare systems in parts of this region can favor therapies that demonstrate robust outcome improvements and cost offsets, while a subset of markets presents opportunities for premium pricing when clear unmet need exists and registry data supports effectiveness. In contrast, the Asia-Pacific region exhibits rapid expansion of diagnostic capacity and increasing investment in local manufacturing, yet variations in reimbursement and infrastructure can create heterogeneity in access timelines and clinical adoption.
These regional differences influence commercial planning, regulatory strategy, and evidence generation priorities. Sponsors must adapt trial designs, post-approval commitments, and market access dossiers to reflect local payer demands and clinical practice patterns. Collaborative initiatives that build regional registries, strengthen rare disease networks, and support capacity building in specialized centers can accelerate appropriate adoption and ensure that therapeutic advances translate into improved patient outcomes across diverse healthcare systems.
Competitive strategies and collaborative models companies employ to convert scientific advances into durable therapies, supply resilience, and payer-ready value propositions
Key companies active in the Gaucher therapy landscape are advancing distinct strategic plays that range from optimizing established biologics to pioneering gene-based curative ambitions and refining oral substrate reduction modalities. Established biologics manufacturers continue to invest in lifecycle management efforts to enhance manufacturing efficiency, improve safety profiles, and support long-term registries that document clinical outcomes. These activities sustain relationships with specialist centers and reinforce supply reliability for patients dependent on established enzyme replacement regimens.Meanwhile, innovators pursuing gene therapy strategies focus on vector engineering, durable expression, and safety monitoring to address both visceral and neurologic disease components. Their programs emphasize long-term follow-up, center-of-excellence partnerships for delivery, and carefully designed post-marketing surveillance. Companies developing orally administered substrate reduction agents concentrate on improving tolerability, simplifying dosing, and demonstrating adherence advantages in outpatient populations. These companies engage with clinicians and patient groups to collect real-world evidence that complements randomized trial data and informs payer dialogues.
Across the competitive landscape, strategic alliances, licensing agreements, and manufacturing partnerships play an essential role in scaling programs and accelerating access. Collaboration between companies with complementary assets-such as pairing a gene delivery platform with a clinical development partner or aligning a substrate reduction developer with an established distribution network-can reduce time-to-access and spread development risk. Ultimately, success depends on translating scientific promise into reproducible clinical benefit, ensuring supply chain resilience, and delivering compelling value propositions to payers, providers, and patients.
Actionable strategic recommendations for industry leaders to secure access, build resilient supply chains, and align evidence generation with payer and clinical needs
Industry leaders must adopt pragmatic, multi-dimensional strategies that accelerate patient access while safeguarding clinical integrity and commercial sustainability. First, prioritize integrated evidence plans that combine randomized controlled trial outcomes with robust real-world data collection; this hybrid approach addresses both regulatory expectations and payer demands, and it supports differentiated value narratives that resonate with clinicians and health systems. Second, allocate resources to supply chain diversification and manufacturing localization where tariff exposure or logistics vulnerabilities threaten continuity of supply, thereby protecting patient access and limiting commercial disruption.Third, design commercialization pathways that reflect segmentation realities: tailor clinical programs and access strategies for distinct patient cohorts such as pediatric versus adult populations, and configure distribution models to match whether hospital specialty pharmacies or community retail channels will optimize adherence and monitoring. Fourth, invest in stakeholder engagement, including early and continuous dialogue with regulatory authorities, payers, and patient advocacy groups, to co-create acceptable endpoints, reimbursement frameworks, and post-approval evidence commitments. Fifth, pursue strategic partnerships that complement internal capabilities, whether through licensing, co-development agreements, or manufacturing alliances that accelerate scalability and minimize capital intensity.
By following these recommendations, organizations can reduce time-to-access, enhance the robustness of their evidence base, and ensure that therapeutic innovations align with real-world clinical workflows. Execution depends on cross-functional coordination among R&D, regulatory affairs, market access, and commercial teams to translate strategic intent into operational reality and to maintain momentum from clinical validation through broad patient adoption.
A rigorous mixed-methods research design combining expert interviews, literature triangulation, and segmentation analysis to produce evidence-aligned strategic insights
The research methodology underpinning this analysis blends primary and secondary approaches to ensure a balanced, evidence-driven perspective. Primary inputs include structured interviews with clinicians, payers, and industry executives, as well as qualitative feedback from patient advocates and specialty pharmacists. These conversations informed interpretation of clinical priorities, access challenges, and real-world practice patterns. Secondary research involved a systematic review of peer-reviewed literature, regulatory guidance documents, clinical trial registries, and company disclosures to synthesize therapeutic mechanisms, safety considerations, and development trends.Analytical methods integrated thematic coding of interview transcripts with cross-validation against published clinical data and regulatory precedent. Segmentation analysis drew on aggregated clinical descriptions and prescribing patterns to differentiate needs across disease types, age groups, and care settings. Regional insights were developed by mapping regulatory frameworks and reimbursement pathways and by triangulating stakeholder input with public policy documents. Risk analysis assessed supply chain vulnerabilities, tariff exposure, and potential regulatory hurdles that could affect timely access.
Limitations of the methodology include the evolving nature of clinical evidence in rare disease spaces and the geographic variability of healthcare delivery that can complicate universal generalizations. To mitigate these factors, the study emphasizes transparency in data sources, relies on multiple independent expert perspectives, and recommends ongoing update cycles to capture new clinical results, regulatory changes, and market developments as they arise.
Concluding synthesis that ties clinical innovation, operational resilience, and stakeholder collaboration to tangible strategies for improving outcomes in Gaucher disease
In conclusion, the therapeutic landscape for Gaucher disease is at an inflection point where oral therapies present tangible advantages in convenience and outpatient management, gene therapies offer transformative potential for durable correction, and enzyme replacement treatments continue to provide a reliable clinical foundation. Stakeholders must navigate a complex interplay of clinical heterogeneity, regulatory expectations, payer scrutiny, and operational constraints to translate innovation into improved patient outcomes. The balance between clinical benefit and delivery feasibility will determine which modalities gain traction in specific patient cohorts and healthcare systems.To succeed, organizations should integrate flexible evidence-generation strategies, resilient supply chain planning, and targeted commercial models that reflect segmentation and regional nuances. Collaboration across industry, clinical communities, and advocacy groups will be essential to accelerate diagnosis, strengthen registries, and ensure equitable access. As therapeutic options expand, the value proposition will hinge not only on efficacy and safety but also on how well interventions reduce treatment burden and align with long-term care pathways.
Ultimately, strategic clarity, operational discipline, and stakeholder engagement will determine whether scientific advances translate into meaningful improvements in quality of life for people living with Gaucher disease. Continued focus on patient-centered metrics and adaptive commercialization will enable the field to convert potential into practical, sustainable care solutions.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- AbbVie Inc.
- Actelion Pharmaceuticals Ltd.
- ADVANZ PHARMA Holdco Limited
- Amicus Therapeutics, Inc.
- Anthera Pharmaceuticals, Inc.
- AstraZeneca plc
- AVROBIO, Inc.
- Enobia Pharma, Inc.
- Freeline Therapeutics Holdings plc
- Genzyme Corporation
- GlaxoSmithKline plc
- Horizon Therapeutics plc
- JCR Pharmaceuticals Co., Ltd.
- Merck & Co., Inc.
- Novartis AG
- Orphazyme A/S
- Pfizer Inc.
- Pharming Group N.V.
- Sanofi S.A.
- Shire Human Genetic Therapies, Inc.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- Ultragenyx Pharmaceutical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
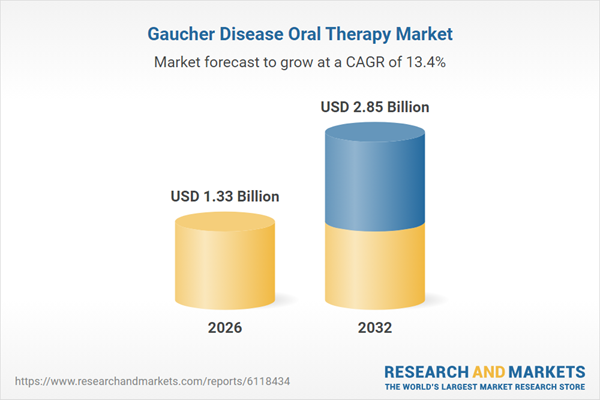
| Estimated Market Value ( USD | $ 1.33 Billion |
| Forecasted Market Value ( USD | $ 2.85 Billion |
| Compound Annual Growth Rate | 13.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |