Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction that frames sterile liquid handling bag evolution across clinical use cases, material innovation, regulatory demands, and procurement priorities
Sterile liquid handling bags now occupy a pivotal role across clinical and laboratory environments, acting as the primary interface where containment, sterility, and biocompatibility converge. Over the last decade, device designers and procurement leaders have elevated expectations beyond simple containment: bags must support complex biologics handling, enable closed-system workflows, and align with increasingly stringent sterilization and material safety standards. These shifts have tightened the link between product design and clinical outcomes, particularly in applications where contamination risk directly affects patient safety or sample integrity.Concurrently, the supply chain and manufacturing paradigms underpinning these products have evolved. Material science developments such as advanced polymer blends and multi-layer film constructions permit thinner, more flexible bags while preserving barrier properties and extractables profiles. Regulatory pathways continue to emphasize validated manufacturing controls, extractables and leachables assessments, and documentation sufficient for device clearance or registration. Consequently, suppliers are investing in higher-grade production lines, enhanced quality systems, and stronger post-market surveillance to meet customer demands.
Looking ahead, the introduction of new clinical applications, intensified focus on single-use systems, and growing attention to lifecycle sustainability will further shape design priorities. Clinicians and procurement teams increasingly value solutions that simplify handling, minimize contamination risk, and integrate seamlessly into existing workflows. As a result, manufacturers that combine material innovation with robust regulatory and supply chain practices will be best positioned to meet the converging needs of hospitals, ambulatory settings, and research facilities.
How clinical innovation, sustainability pressures, evolving channels, and geopolitical shifts are driving a rapid transformation of sterile liquid handling bag development and supply strategies
Recent transformative shifts in the sterile liquid handling bag landscape reflect a confluence of clinical innovation, supply chain reorientation, and regulatory tightening that together are reshaping product development and commercialization strategies. First, clinical demand patterns have become more nuanced as therapies such as cell and gene treatments require single-use, closed-system containers with exceptional biocompatibility and traceability. This has raised the bar for material selection, sterility assurance, and supply chain transparency, prompting manufacturers to adopt advanced polymer technologies and invest in validated manufacturing processes.Second, sustainability and circular-economy considerations have moved from peripheral talking points to procurement criteria in several healthcare systems. As a result, manufacturers are exploring recyclable or lower-carbon material pathways, thinner film architectures that reduce material use, and production efficiencies that cut waste. Meanwhile, distribution models are fragmenting: direct sales channels and online procurement platforms are gaining traction alongside traditional distributor networks, forcing suppliers to re-evaluate channel strategies and customer engagement models.
Finally, geopolitical and trade developments have accelerated supplier diversification and nearshoring initiatives. Companies are increasingly balancing cost pressures with the need for resilient supply lines, shortening lead times, and ensuring local regulatory compliance. Taken together, these shifts compel industry participants to align R&D priorities with operational resilience and to foreground compliance, sustainability, and user-centered design in order to remain competitive.
An authoritative analysis of how the 2025 United States tariff adjustments have reshaped sourcing, manufacturing localization, and commercial contracting across the sterile liquid bag value chain
The cumulative impact of the United States tariff adjustments announced in 2025 extends beyond headline cost increases to influence strategic sourcing, supplier relationships, and product architecture across the sterile liquid handling bag ecosystem. Tariff-induced cost pressure has prompted procurement teams to reassess sourcing footprints and to explore alternative material suppliers in regions with more favorable trade terms. Many manufacturers responded by accelerating dual-sourcing strategies and by qualifying secondary suppliers to mitigate import-related bottlenecks and ensure continuity for clinical customers.Moreover, the tariffs have nudged innovation pathways. To offset direct cost increases on imported raw materials, some manufacturers prioritized development of lower-cost polymer formulations and multi-layer constructions that reduce reliance on single imported components. Others shifted production closer to key end markets to lower landed costs and improve responsiveness to demand fluctuations. This localization trend often carries secondary benefits such as shortened validation cycles and faster regulatory submissions due to more accessible technical documentation and closer proximity to clinical partners.
From a distribution standpoint, the tariffs have encouraged greater transparency in cost pass-through mechanisms and more collaborative pricing discussions between suppliers and large institutional buyers. Hospitals and large buying groups are negotiating longer-term agreements and exploring inventory management solutions that stabilize supply and reduce exposure to sudden tariff shifts. In sum, the 2025 tariff environment has catalyzed structural responses across sourcing, product design, and commercial contracting that will influence strategic planning well beyond the initial policy window.
Deep segmentation insights that link material choices, clinical applications, end-user needs, capacity requirements, and distribution channels to strategic product differentiation and commercialization imperatives
Segmentation insight reveals differentiated demand drivers and strategic priorities across material groups, applications, end users, capacities, and distribution pathways that together define product development and go-to-market choices. Materials divide into two primary schools: PVC and non-PVC. Within the non-PVC category, multi-layer film architectures, polyethylene (PE), and polypropylene (PP) variants each offer distinct trade-offs in flexibility, barrier performance, and extractables profiles, which in turn influence suitability across sterilization methods and biologic compatibility requirements.Application-level distinctions further shape product specification. Blood collection and cell culture workflows impose stringent sterility and extractables controls; drainage, infusion, and irrigation bags prioritize flow characteristics, closure integrity, and compatibility with existing lines and pumps. End-user contexts also drive design priorities: ambulatory surgical centers and clinics typically demand compact, easy-to-handle formats and rapid supply replenishment, whereas hospitals and research laboratories may prioritize batch traceability, scalability, and compatibility with specialized instrumentation.
Capacity segmentation intersects with clinical workflow preferences and inventory management. Smaller bags less than 500 milliliters serve ambulatory procedures and specific lab workflows, mid-range capacities between 500 and 1000 milliliters address common infusion and collection needs, and larger formats over 1000 milliliters support bulk fluid management in clinical and research contexts. Distribution channel dynamics complete the picture: direct sales relationships, distributor networks, online procurement platforms, and retail pharmacies each require tailored packaging, documentation, and service-level commitments to meet customer expectations across the ecosystem.
Regional dynamics and cross-border regulatory trends shaping demand, sustainability expectations, and supply chain strategies for sterile liquid handling bags across major global markets
Regional dynamics shape demand patterns, regulatory expectations, and supply chain strategies in distinct ways that affect producers and purchasers of sterile liquid handling bags. In the Americas, hospitals and a growing network of ambulatory surgical centers prioritize robust supply continuity, validated quality systems, and close regulatory alignment with domestic authorities. Procurement organizations in this region increasingly favor suppliers that can demonstrate traceability, rapid response capabilities, and strong post-market support for adverse event management.In Europe, the Middle East, and Africa, regulatory heterogeneity and heightened emphasis on sustainability create both challenges and opportunities. European buyers often demand stringent environmental reporting alongside traditional quality credentials, pressuring manufacturers to provide lifecycle assessments and evidence of reduced environmental impact. Meanwhile, markets in the Middle East and Africa vary widely in procurement sophistication, presenting growth opportunities for suppliers able to combine compliant documentation with flexible commercial models and localized distribution partnerships.
Asia-Pacific remains both a major manufacturing hub and a rapidly expanding demand center. Strong domestic manufacturing capabilities in several countries support competitive production economics, while rising clinical adoption and expansion of research infrastructure drive local demand for specialized bag formats, particularly for cell culture and infusion. Across all regions, cross-border regulatory convergence and increased emphasis on supplier transparency are motivating firms to invest in harmonized technical documentation and to pursue regional qualification strategies that reduce time to market and improve customer trust.
Insights into how technological differentiation, strategic partnerships, and customer-centric commercial models determine competitive advantage among sterile liquid handling bag suppliers
Competitive dynamics in the sterile liquid handling bag space are defined by firms that combine material science expertise, validated manufacturing processes, and channel agility. Market leaders typically differentiate through investments in proprietary film technologies, rigorous extractables and leachables testing programs, and strong quality management systems that facilitate regulatory compliance and customer trust. Strategic partnerships with contract manufacturers and sterilization providers also play a crucial role in enabling scale and flexibility, particularly when new clinical applications require rapid qualification of customized bag formats.Recent patterns show greater convergence between product innovation and commercial model innovation. Companies that pair differentiated technical capabilities with flexible commercial terms and digital services such as lot-level traceability portals or product configurators gain preferential consideration from large institutional buyers. In addition, consolidation activity and strategic alliances have accelerated specialization in high-growth application niches like cell culture, where requirements for material performance and contamination control are most exacting. Supplier reputations for responsiveness, technical support during clinical validation, and post-market surveillance increasingly determine customer selection, especially for high-stakes clinical and laboratory applications.
Finally, nimble smaller players continue to influence the landscape by focusing on niche formats and rapid customization, compelling larger firms to incorporate modular production and faster product development cycles to maintain competitive parity. Overall, the interplay of technological differentiation, partnerships, and customer-centric commercial models defines the current competitive environment.
Actionable recommendations for manufacturers, distributors, and health systems to diversify supply, accelerate material innovation, modernize commercialization, and strengthen regulatory resilience
Industry leaders can take a set of immediate, actionable steps to strengthen competitive positioning, protect margins, and better serve clinical and laboratory customers. First, diversify upstream supply bases and qualify secondary suppliers, prioritizing local or regional partners where feasible to reduce exposure to trade disruptions and tariff volatility. Simultaneously, invest in material substitution programs that evaluate non-PVC multi-layer constructions and high-performance PE and PP blends to balance cost, performance, and regulatory acceptability.Second, align product development roadmaps with clinical workflow trends by collaborating closely with end users during design validation. Co-development agreements with major hospital systems and research laboratories can shorten adoption cycles and provide real-world performance data that support regulatory submissions. Third, modernize commercial approaches by integrating direct sales efforts with digital ordering platforms and by offering value-added services such as inventory management solutions, lot-level traceability, and technical onboarding support.
Fourth, incorporate lifecycle sustainability metrics into product positioning and procurement documentation to address growing environmental criteria among institutional buyers. Finally, prioritize regulatory rigor and transparency by standardizing technical dossiers across regions and by investing in post-market surveillance capabilities that demonstrate safety and reliability. These measures, when combined, create a resilient operational foundation and foster trust across hospital, clinic, and laboratory customers.
A transparent research methodology blending expert primary interviews, technical literature review, patent and regulatory analysis, and data triangulation to support rigorous insights
This research synthesizes primary and secondary inputs through a structured methodology designed to ensure validity, reproducibility, and practical relevance. Primary research included interviews with subject-matter experts across clinical procurement, product development, regulatory affairs, and distribution management to capture first-hand observations about material performance, sterilization compatibility, and sourcing priorities. These stakeholder perspectives were used to ground product-level insights and to surface operational responses to recent trade and regulatory developments.Secondary research involved review of peer-reviewed literature, regulatory guidance documents, patent filings, and industry technical publications to map material technologies, sterilization practices, and clinical use cases. Technical validation steps included cross-referencing product specifications with sterilization compatibility studies and extractables and leachables literature to ensure material assertions are supported by empirical data. Data triangulation practices combined qualitative interview findings with documentary evidence to reduce bias and to strengthen inference.
Throughout the process, quality controls involved independent review cycles, expert validation, and consistency checks across regions and application areas. Limitations of the methodology are acknowledged, including the evolving nature of clinical applications and the potential for rapid policy changes that can alter supply chain dynamics. Nevertheless, the approach emphasizes transparency, rigorous validation, and actionable synthesis aimed at informing strategic decisions.
A concise conclusion highlighting how integrated material innovation, supply chain resilience, and commercial strategy will determine future success in the sterile liquid handling bag market
In conclusion, the sterile liquid handling bag landscape stands at an inflection point where material innovation, regulatory discipline, and supply chain strategy converge to determine competitive advantage. The combined pressures of evolving clinical needs-especially in cell therapy and closed-system biologic workflows-sustainability expectations, and trade-related cost dynamics require manufacturers and purchasers to adopt a more integrated approach to product design, sourcing, and commercialization. Firms that proactively diversify supply bases, invest in validated non-PVC alternatives, and strengthen regulatory and post-market capabilities will be better equipped to respond to shifting procurement criteria and clinical demands.Moreover, the interplay between distribution models and end-user preferences calls for tailored commercial strategies that blend direct engagement with digital procurement options and distributor partnerships. Regional considerations further complicate but also create opportunities for strategic localization and differentiated value propositions. Ultimately, this body of insight underscores the importance of aligning technical, operational, and commercial actions to deliver sterile liquid handling bag solutions that satisfy clinical performance requirements while managing cost, compliance, and environmental responsibilities.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Sterile Liquid Handling Bag Market
Companies Mentioned
- ABEC Inc
- Agilent Technologies Inc
- Avantor Inc
- B. Braun Melsungen AG
- Baxter International Inc
- Becton Dickinson and Company
- Corning Incorporated
- Danaher Corporation
- Entegris Inc
- Eppendorf SE
- Fresenius Kabi AG
- Fujimori Kogyo Co Ltd
- GEA Group AG
- Gilson Inc
- Grifols S.A.
- Hamilton Company
- ICU Medical Inc
- Lonza Group AG
- Meissner Filtration Products Inc
- Merck KGaA
- Nipro Corporation
- Poly Medicure Limited
- Repligen Corporation
- Saint-Gobain
- Sartorius AG
- Single Use Support GmbH
- Tecan Group Ltd
- Terumo Corporation
- Thermo Fisher Scientific Inc
- VWR International LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
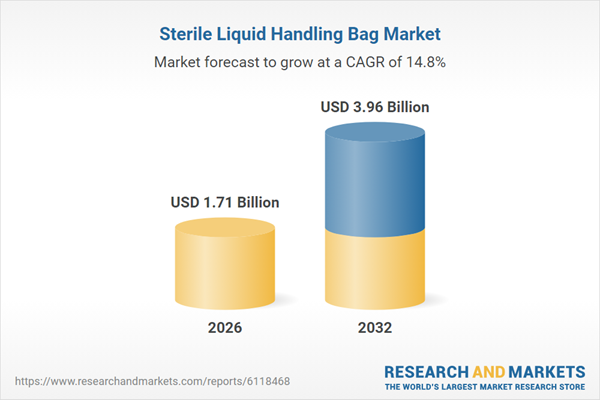
| Estimated Market Value ( USD | $ 1.71 Billion |
| Forecasted Market Value ( USD | $ 3.96 Billion |
| Compound Annual Growth Rate | 14.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |