Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive introduction to how additive manufacturing is converging with clinical workflows to deliver patient-specific solutions and system-level value across healthcare settings
The medical 3D printing landscape is entering a phase where clinical utility and manufacturing capability converge to reshape patient care pathways and device production. Advanced additive technologies now enable increasingly complex geometries and patient-specific solutions, while improvements in materials and post-processing expand clinical applicability across dental restorations, implants, prosthetic limbs, and surgical guides. Clinical teams, device manufacturers, and research organizations are therefore aligning around three core objectives: improving clinical outcomes, accelerating time-to-treatment, and reducing overall production complexity.Consequently, stakeholders are adopting a systems perspective that emphasizes interoperability between design software, printing hardware, material supplies, and quality assurance workflows. This systems approach is driving institutional investments in in-house capabilities at dental clinics and hospitals as well as partnerships between medical device manufacturers and academic or corporate research units. As a result, the ecosystem is evolving from a tool-centric model to an outcomes-driven model, where technology selection and process design are evaluated through clinical validation and regulatory readiness.
Transitioning from pilot projects to routine clinical use requires robust evidence generation and repeatable manufacturing protocols. Therefore, interdisciplinary collaboration among clinicians, biomedical engineers, regulatory experts, and supply chain managers has become a prerequisite for successful adoption. In sum, the market’s maturity is reflected in higher expectations for end-to-end solutioning, amplified collaboration across stakeholder groups, and an emphasis on scalable, validated processes that support consistent patient outcomes
How advances in machine precision, material diversity, regulatory maturation, and integrated manufacturing networks are reshaping clinical adoption and supplier strategies
The shifts underway in medical 3D printing reflect a combination of technological refinement and strategic repositioning across the healthcare value chain. On the technological front, high-resolution stereolithography and selective laser sintering are enabling finer anatomical fidelity for surgical planning and implantable devices, while binder jetting and material extrusion are increasing throughput for larger-volume components and prosthetic constructs. At the same time, material science advances in ceramics, metals, composites, and polymers are broadening the palette of functional, biocompatible, and sterilizable options that clinicians can rely on for specific indications.From a strategic perspective, the industry is moving away from isolated pilot deployments toward integrated manufacturing networks that balance centralized specialty fabrication with distributed, near-patient production. This trend is driven by the need to shorten logistics cycles, improve responsiveness for personalized devices such as dental aligners and crowns, and provide hospitals with the agility to produce surgical planning models and orthotics on demand. Moreover, regulatory frameworks and reimbursement dialogues are maturing, which prompts manufacturers and clinical adopters to invest in quality systems, validated workflows, and evidence dossiers to support broader clinical acceptance.
Consequently, the competitive landscape is also evolving as pure-play equipment suppliers, material innovators, and medical device manufacturers form strategic alliances to offer bundled solutions that reduce adoption friction. As a result, purchasers increasingly evaluate vendors on the basis of total solution value - encompassing hardware, validated materials, software interoperability, and post-sale services - rather than on individual product features alone
Assessing how tariff-driven trade policy shifts interact with supply chain resilience, procurement behavior, and regional production strategies across the medical additive manufacturing ecosystem
The imposition of tariffs and shifting trade policy dynamics have cumulative effects that extend across equipment procurement, material sourcing, and international collaboration within the medical 3D printing ecosystem. Tariff measures influence the relative cost of imported printers, spare parts, and raw materials including metals and specialty polymers, which in turn affects purchasing strategies for hospitals, dental clinics, and medical device manufacturers. Consequently, procurement teams respond by reallocating budgets, prioritizing total cost-of-ownership assessments, and scrutinizing supplier footprints to mitigate tariff exposure.In parallel, tariffs alter the incentives for onshoring or nearshoring production capabilities. Manufacturers and research institutions weigh the benefits of localized fabrication against the capital and operational expenses associated with establishing domestic supply chains. As a result, some actors accelerate investments in in-country production of critical components and validated materials, while others diversify supplier networks across tariff-exempt jurisdictions and negotiate long-term agreements to lock in more predictable input costs.
Moreover, non-tariff trade policy responses and customs procedures add administrative friction that can prolong lead times for specialized consumables and replacement parts. This creates pressure to strengthen inventory strategies, improve demand forecasting, and formalize contingency plans for clinical continuity. Interdisciplinary stakeholders therefore emphasize robust supplier qualification processes and supply chain traceability to ensure sustained access to biocompatible materials and regulatory-compliant components. Ultimately, the cumulative impact of tariff policy manifests as a strategic catalyst for supply chain resilience, regional capacity building, and more stringent vendor evaluation criteria
Insightful segmentation that aligns application needs, technological capabilities, material characteristics, and end-user operational priorities to reveal differentiated adoption pathways
Segmentation analysis clarifies where clinical need, technological capability, material properties, and end-user priorities intersect to create differentiated opportunities across the ecosystem. When considering application-driven demand, dental workflows spanning aligners, bridges, and crowns present steady requirements for high-precision polymer and composite solutions, whereas implant manufacturing and surgical planning call for metals and ceramics that meet stringent biocompatibility and sterilization standards. Prosthetic applications, including both limbs and orthotics, prioritize customizable geometries and durable composites or polymers to balance comfort with mechanical resilience.In parallel, technology selection shapes production strategy: binder jetting, including its ceramic and metal variants, offers throughput advantages for complex batch builds; stereolithography delivers surface quality and resolution suited to dental and surgical models; selective laser sintering provides material versatility for functional parts; and material extrusion supports cost-effective prototyping and larger-format prosthetics. Each technological approach requires matching material systems - whether ceramics, composites, metals, or polymers - that bring distinct processing parameters, post-processing needs, and validation requirements.
Finally, end-user segmentation underlines the operational constraints and adoption drivers that differ between dental clinics, hospitals, medical device manufacturers, and research institutions. Dental clinics emphasize workflow integration, turnaround speed, and patient-facing aesthetics; hospitals focus on regulatory compliance, sterilizability, and clinical outcomes; device manufacturers require scalable, repeatable processes and supplier control; and research institutions, spanning academic labs and corporate research, drive innovation, method validation, and early-stage material testing. Together, these dimensions illustrate how application, technology, material, and end-user considerations must be aligned to design commercially viable and clinically effective solutions
A comparative regional perspective describing how Americas, Europe Middle East & Africa, and Asia-Pacific dynamics influence adoption, regulation, and manufacturing strategies
Regional dynamics shape competitive positioning, supply chain design, and clinical adoption patterns in the medical 3D printing industry. In the Americas, healthcare providers and device developers push for near-patient production capabilities and rapid clinical validation, often leveraging academic-industry partnerships to accelerate translation from prototype to clinical use. This orientation encourages investment in localized manufacturing capacity, regulatory expertise, and training programs to support point-of-care applications within hospitals and dental clinics.Meanwhile, Europe, Middle East & Africa exhibit a heterogeneous landscape where regulatory stringency, public procurement practices, and fragmented reimbursement models create both barriers and targeted opportunities. In some European markets, strong regulatory frameworks and established quality systems incentivize manufacturers to pursue rigorous clinical evidence and certification pathways, while in certain EMEA regions, partnerships with local research institutions and regional manufacturers help bridge capability gaps and expand service provision.
Across Asia-Pacific, rapid adoption is driven by a combination of government-backed innovation programs, investments in additive manufacturing infrastructure, and growing private healthcare demand. Large hospital networks and medical device companies in the region often emphasize scalable production and cost optimization, which favors technology choices that balance throughput with material performance. Consequently, regional strategies frequently include collaborative ventures and localized material development to meet specific clinical and commercial requirements. Across all regions, stakeholders prioritize supply chain resilience, regulatory alignment, and workforce development to translate technological promise into sustained clinical impact
How hardware innovators, material scientists, software integrators, and service-oriented providers are strategically partnering to deliver validated, clinic-ready additive manufacturing solutions
Competitive dynamics in the sector are characterized by a blend of hardware innovation, material science leadership, software integration, and service-oriented business models. Leading equipment manufacturers differentiate through printing resolution, repeatability, and validated workflows for clinical applications, while material suppliers compete on biocompatibility, sterilization compatibility, and batch consistency. Software and workflow platforms play an increasingly decisive role by enabling scan-to-print interoperability, automating design for additive manufacturing, and providing traceability to support regulatory compliance.Additionally, medical device companies and contract manufacturers position themselves as end-to-end solution providers by integrating design services, validated materials, and post-processing capabilities to reduce adoption friction for clinical customers. Research institutions and academic labs contribute by generating clinical evidence and pilot data that de-risk technology adoption, and corporate R&D units accelerate commercial translation through targeted collaborations. Consequently, strategic partnerships, licensing arrangements, and co-development agreements have become common as firms seek to combine complementary strengths and accelerate route-to-clinic.
Investors and corporate development teams therefore evaluate potential partners not only for their technological performance, but also for their quality systems, regulatory track record, and ability to scale production under healthcare-grade controls. This multi-dimensional view of competitive advantage reshapes how companies allocate R&D budgets, structure go-to-market strategies, and prioritize clinical validation initiatives
Actionable recommendations for leaders to strengthen clinical validation, diversify supply chains, and integrate interoperable workflows to accelerate adoption and scale
Industry leaders should adopt an integrated approach that addresses clinical validation, supply chain resilience, and scalable operations to capitalize on evolving demand. First, prioritize investments in clinically-focused evidence generation and quality management systems that align with regulatory expectations for dental applications, implantable devices, prosthetic constructs, and surgical planning models. By building robust validation pathways, organizations can reduce adoption friction and accelerate integration into clinical workflows.Second, diversify supplier relationships and consider regional production or strategic partnerships to mitigate tariff exposure and logistics disruptions. Nearshoring select manufacturing activities, qualifying multiple material sources across ceramics, metals, composites, and polymers, and establishing contingency inventory policies will support continuity for hospitals and dental clinics that rely on timely delivery. Third, enhance software and workflow interoperability to streamline scan-to-print processes across stereolithography, selective laser sintering, binder jetting, and material extrusion platforms, thereby lowering operational complexity for end users.
Finally, cultivate cross-sector collaborations with academic labs, corporate research units, and clinical champions to co-develop validated applications and training programs. This collaborative posture accelerates clinical evidence generation, expands the talent pipeline, and builds trust with purchasers. Taken together, these priorities enable leaders to offer comprehensive value propositions that combine validated technology, dependable supply chains, and scalable operations to meet diverse end-user needs
A rigorous, multi-method research methodology combining expert interviews, technical assessments, regulatory reviews, and regional supply chain analysis to validate findings
The research approach combines qualitative stakeholder interviews, technology capability assessments, and cross-functional process mapping to ensure robust, reproducible conclusions. Primary input was gathered through structured conversations with clinicians, biomedical engineers, device manufacturers, and procurement specialists to capture real-world challenges in clinical workflows and supply chain operations. These insights were triangulated with technical evaluations of printer platforms, material properties, and post-processing requirements to assess suitability for dental restorations, implants, prosthetics including limbs and orthotics, and surgical planning models.In addition, the methodology incorporated systematic review of regulations, standards, and clinical guidelines to understand the evidence and certification expectations that affect adoption across hospitals, dental clinics, and manufacturers. Technology comparisons considered binder jetting variants such as ceramic and metal binder jetting, stereolithography, selective laser sintering, and material extrusion, while material analyses examined ceramics, composites, metals, and polymers with respect to biocompatibility and process constraints. Finally, regional supply chain and policy analysis informed scenario planning for Americas, Europe Middle East & Africa, and Asia-Pacific contexts.
Throughout the study, findings were validated with domain experts and cross-checked for consistency across multiple data sources, ensuring that conclusions reflect current technological capabilities, operational realities, and regulatory considerations. This rigorous, multi-method approach supports actionable insights that stakeholders can apply to strategic planning and operational decision-making
Concluding synthesis emphasizing the necessity of validated processes, material-technology alignment, and supply chain resilience to capture clinical and commercial value
The trajectory of medical 3D printing is defined by the intersection of material innovation, precision equipment, validated clinical workflows, and strategic supply chain design. As technology continues to mature, stakeholders must prioritize clinical validation, interoperable workflows, and resilient sourcing strategies to translate technical capability into reliable patient outcomes. Dental applications such as aligners, bridges, and crowns will continue to benefit from high-resolution polymer and composite solutions, while implant manufacturing and surgical planning demand metals and ceramics that meet stringent clinical requirements.Moreover, technology choices from binder jetting variants to stereolithography, selective laser sintering, and material extrusion each bring unique trade-offs in resolution, throughput, and material compatibility, necessitating careful alignment with application needs and end-user constraints. Hospitals, dental clinics, manufacturers, and research institutions each have distinct operational drivers that influence adoption pathways, making tailored solutions and partnership models increasingly important.
In closing, success in this dynamic sector depends on a strategic balance between innovation and operational rigor. Organizations that invest in validated processes, diversify supply relationships across regions, and foster collaborative research partnerships will be best positioned to convert promising technologies into repeatable clinical value and durable competitive advantage
Table of Contents
17. ResearchStatistics
18. ResearchContacts
19. ResearchArticles
20. Appendix
Companies Mentioned
- 3D Systems Corporation
- 3DHealer
- 3T Additive Manufacturing Ltd.
- Arcam AB
- Aspect Biosystems Ltd.
- Biomedical Modeling, Inc.
- Bio-Rad Laboratories, Inc.
- CELLINK AB
- Cyfuse Biomedical K.K.
- Desktop Metal, Inc.
- EnvisionTEC GmbH
- EOS GmbH
- GE Additive
- HP Inc.
- LimaCorporate
- Materialise NV
- Meril Life Sciences Pvt. Ltd.
- Poietis SAS
- Protolabs, Inc.
- RegenHU Ltd.
- Renishaw plc
- Rokit Healthcare
- SLM Solutions Group AG
- Stratasys Ltd.
- TeVido BioDevices, LLC
- Xilloc Medical B.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
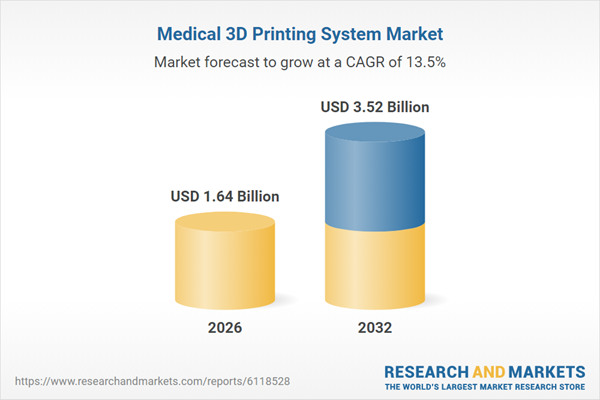
| Estimated Market Value ( USD | $ 1.64 Billion |
| Forecasted Market Value ( USD | $ 3.52 Billion |
| Compound Annual Growth Rate | 13.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |