Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative overview of pharmaceutical-grade potassium chloride priorities linking purity control supply continuity and regulatory compliance for product developers
Pharmaceutical-grade potassium chloride occupies a critical position at the intersection of clinical safety, supply chain integrity, and formulation science. As manufacturers refine electrolyte formulations for oral and parenteral therapies, purity thresholds, particulate control, and source traceability have become non-negotiable aspects of product development and regulatory submissions. Concurrently, tightening regulatory scrutiny around excipient provenance and manufacturing controls has elevated the strategic importance of high-purity inorganic salts.In practice, procurement teams must balance technical specifications against continuity of supply, while development scientists require consistent physico-chemical performance across batches to minimize downstream variability in stability and bioavailability. This dynamic places potassium chloride suppliers under increasing pressure to demonstrate robust quality systems, validated analytical methods, and transparent supply chains. Moreover, collaboration between quality, regulatory, and sourcing functions is now essential to ensure that raw material decisions support both product efficacy and compliance across global markets.
Taken together, these forces are reshaping how pharmaceutical-grade potassium chloride is evaluated and integrated into therapeutic products. The following executive summary synthesizes key shifts, tariff implications, segmentation nuances, regional considerations, competitive behaviors, and recommended actions to help decision-makers align supply strategies with clinical and commercial objectives.
How evolving analytical standards digital traceability and strategic sourcing are reshaping supplier qualification and continuity for high-purity pharmaceutical salts
The landscape for pharmaceutical-grade potassium chloride is in the midst of transformative shifts driven by technology adoption, regulatory tightening, and strategic sourcing changes. Analytical enhancements, including higher-resolution impurity profiling and validated limit-testing methodologies, have raised the bar for supplier qualification. These advancements enable more precise differentiation between high-purity and standard-purity grades, which in turn informs formulation choices for injectable and oral dosage forms. As a result, pharmaceutical developers are increasingly specifying narrow acceptance criteria and demanding full documentation of analytical traceability from manufacturers.At the same time, digital tools and data transparency are reshaping supplier relationships. Blockchain pilots and digital batch passports are enabling end-to-end traceability for critical raw materials, reducing the friction associated with audits and change controls. These capabilities are particularly consequential for prefilled syringes and vials where sterile manufacturing environments require unimpeachable material provenance. Transitioning from manual to automated reconciliation of certificates of analysis shortens qualification cycles and reduces supply disruption risk.
Sustainability and geopolitical considerations are also prompting strategic diversification of sources. Manufacturers are exploring regional supply hubs and multi-supplier strategies to mitigate concentration risk while preserving quality. This shift is coupled with a move toward tighter contractual frameworks that embed quality metrics, contingency planning, and penalty clauses to ensure continuity under stress. Collectively, these trends are changing procurement, quality assurance, and regulatory engagement practices and will define competitive advantage over the coming years.
The multifaceted consequences of 2025 US tariff measures on supply chain resilience procurement economics and regulatory pathways for excipient sourcing
The imposition of tariffs and trade restrictions by the United States in 2025 has exerted a cumulative influence across raw material sourcing, manufacturing economics, and supply chain design for pharmaceutical-grade potassium chloride. Increased duties on specific import lines have prompted downstream manufacturers to reassess their supplier mix, favoring closer geographic sourcing or validated domestic producers to reduce landed cost volatility. This adjustment has not only affected procurement cost structures but has also reshaped logistics planning and inventory strategies across production networks.From a manufacturing perspective, tariffs have accentuated the value of securing multi-jurisdictional supplier relationships and building redundancy into qualification protocols. Companies with flexible supplier qualification systems were better positioned to pivot when tariff-impacted shipments became less viable. In parallel, the need to maintain uninterrupted supply of excipients for critical products led many organizations to increase safety stock levels and to adopt more dynamic hedging strategies for procurement contracts. These measures carried trade-offs in working capital and warehousing requirements, requiring finance and operations to collaborate more tightly with procurement and quality teams.
Regulatory interactions also adjusted in response to sourcing shifts. When manufacturers switched raw material origins, the impact on stability data, impurity profiles, and regulatory filings required timely change-control documentation and in some cases preemptive dialogue with health authorities. Consequently, cross-functional teams that included regulatory affairs, quality, and sourcing became essential for managing tariff-driven changes without jeopardizing product availability. Looking forward, the 2025 tariff environment has accelerated resilience planning and highlighted the importance of contractual and operational flexibility in excipient supply chains.
Deep segmentation-driven perspectives revealing how application form end user distribution pathways and purity bands determine sourcing and qualification imperatives
Segmentation analysis reveals how application, form, end user, distribution channel, and purity grade jointly define strategic priorities for pharmaceutical-grade potassium chloride. Based on Application, market considerations span Food And Beverage, Industrial, and Pharmaceutical, with the Pharmaceutical segment further divided into Injectable and Oral Dosage; within Injectable, Prefilled Syringe and Vial categories carry distinct sterility and particulate control demands, while Oral Dosage subdivides into Capsules, Liquid Suspension, and Tablets each of which imposes different dissolution and excipient compatibility criteria. Based on Form, suppliers and formulators address Crystal, Granule, Liquid, and Powder formats, with the Liquid form further separated into Concentrate and Solution options that influence handling, dosing precision, and cold-chain requirements. Based on End User, the landscape includes Contract Research Organizations, Hospitals And Clinics, and Pharmaceutical Manufacturers, and within manufacturers the split between Branded and Generic operations affects procurement horizon and batch-to-batch consistency expectations. Based on Distribution Channel, products reach end users through Direct Sales, Distributors, and Online platforms, while Distributors themselves operate as Chemical Distributors or Pharma Distributors with differing regulatory practices and customer support profiles. Based on Purity Grade, technical differentiation exists between Pharma Grade High Purity and Pharma Grade Standard Purity, with the High Purity category typified by ≥99.5% specifications and the Standard Purity by ≥99% thresholds that influence downstream testing burdens and formulation risk assessments.These segmentation axes interact in ways that influence supplier selection, qualification timelines, and operational readiness. For example, injectable applications supplied as prefilled syringe components typically require high-purity crystal or finely milled powder with rigorous particulate specifications, and they are most commonly procured via direct sales or pharma-specialized distributors by branded pharmaceutical manufacturers with long qualification cycles. Conversely, generic tablet producers might favor granule or powder formats with slightly broader purity tolerances, relying more on distributor relationships and standardized certificates of analysis. Similarly, liquid concentrates or solutions are often preferred by hospitals and clinics for immediate-use preparations, prompting different shelf-life and stability support from manufacturers. Understanding how these segmentation layers align with organizational priorities is essential for tailoring supply agreements, analytical support packages, and contingency plans to the specific risks and performance criteria of each application.
Regional supply chain realities and regulatory nuances across the Americas Europe Middle East & Africa and Asia-Pacific that influence sourcing and quality strategies
Regional dynamics shape both the sourcing decisions and the operational realities for pharmaceutical-grade potassium chloride across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, a strong emphasis on regulatory alignment with stringent pharmacopeial standards and growing interest in near-shore manufacturing have encouraged manufacturers to prioritize suppliers that can demonstrate consistent high-purity production, local regulatory familiarity, and rapid logistics. This region also exhibits a growing preference for contractual safeguards against supply interruptions, driving investment in secondary sourcing and inventory strategies.Europe, Middle East & Africa presents a heterogeneous environment where regulatory requirements vary by jurisdiction but trend toward rigorous impurity controls and full traceability. Manufacturers serving the EMEA region often require additional documentation for cross-border shipments and favor suppliers that maintain comprehensive quality management systems, multilingual documentation, and regional distribution footprints to reduce lead times. In many jurisdictions, environmental compliance and sustainable sourcing considerations further influence supplier selection and long-term partnership strategies.
Asia-Pacific combines robust production capacity with geopolitical and quality assurance considerations that influence global sourcing strategies. Suppliers in this region offer competitive manufacturing scale, but buyers increasingly demand third-party audits, transparent raw-material pedigree, and adherence to international quality standards. The Asia-Pacific market also leads in specialized logistics solutions for liquid concentrates and sterile supply chains, encouraging multinational manufacturers to adopt hybrid sourcing approaches that blend regional cost advantages with stringent quality controls. Across all regions, alignment between regulatory affairs, procurement, and quality functions remains critical to ensure that regional sourcing decisions support global product consistency and patient safety.
How supplier investments in analytics traceability and regional capacity are redefining competitive advantage and buyer relationships in pharmaceutical supply chains
Competitive behavior among suppliers of pharmaceutical-grade potassium chloride is taking on a strategic character as manufacturers seek partners that combine technical capability with operational resilience. Leading suppliers are investing in advanced analytical capabilities and third-party certifications to substantiate high-purity claims while simultaneously expanding their traceability systems to support regulatory submissions and audits. These technical investments are complemented by supply-side measures, including geographically diversified manufacturing nodes, enhanced inventory visibility, and digital batch documentation to shorten qualification times for customers.At the buyer level, pharmaceutical manufacturers differentiate procurement approaches between branded and generic product lines. Branded manufacturers tend to emphasize long-term partnerships, joint development agreements, and tighter specification windows that reduce variability in clinical performance. Generic manufacturers prioritize cost-efficiency and supplier reliability but are increasingly adopting stricter due diligence processes due to regulatory expectations. Contract research organizations and hospitals prioritize rapid access and clear stability support, favoring suppliers that offer ready-to-use solutions such as concentrates and sterile solution formats accompanied by robust stability and handling data.
Distribution strategies are evolving in parallel, with pharma-focused distributors offering value-added services such as regulatory documentation support, cold-chain logistics, and custom packaging. Chemical distributors continue to serve industrial applications but are upgrading service portfolios to meet pharmaceutical buyer needs. Overall, the competitive landscape rewards suppliers capable of delivering end-to-end assurance-from validated manufacturing and analytical rigor to flexible logistics and customer-centric documentation packages.
Practical strategic initiatives procurement quality and regulatory leaders should implement immediately to fortify supply continuity reduce risk and accelerate approvals
Industry leaders should pursue a set of focused actions that strengthen supply resilience, reduce regulatory friction, and optimize total cost of ownership for pharmaceutical-grade potassium chloride. First, integrate tighter technical specifications into supplier selection criteria and require robust analytical evidence that substantiates purity and impurity control. This reduces downstream stability risk and shortens regulatory review cycles. Second, implement multi-source qualification frameworks that permit rapid supplier substitution without compromising product quality; this entails harmonized testing protocols, aligned acceptance criteria, and established audit reciprocity agreements.Third, increase investment in digital traceability and batch-level documentation to accelerate change-control approvals and to improve transparency during audits. Fourth, update contractual terms to include service-level agreements for continuity, contingency clauses tied to tariff or geopolitical shifts, and shared risk mechanisms for critical supply disruptions. Fifth, align procurement, quality, and regulatory teams through joint governance forums that meet regularly to assess supplier performance, approve change controls, and coordinate responses to external shocks. Finally, prioritize engagement with distributors that offer pharmaceutical-grade logistics, regulatory support, and packaging flexibility to reduce lead times and handling risk. Together, these actions provide a practical roadmap to maintain both product quality and supply continuity while enabling cost-effective operations.
A rigorous mixed-methods approach combining practitioner interviews technical document review and supply chain mapping to produce actionable intelligence for stakeholders
The research behind this analysis deployed a mixed-methods approach combining primary interviews, technical document review, and systems-level supply chain analysis. Primary inputs included structured interviews with procurement leaders, regulatory affairs professionals, formulation scientists, and distribution specialists to capture cross-functional perspectives on quality requirements, qualification timelines, and logistics complexities. These qualitative insights were complemented by technical reviews of pharmacopeial monographs, stability testing protocols, and standard operating procedures to contextualize purity and analytical expectations.Supply chain analysis incorporated mapping of supplier footprints, logistics corridors, and tariff exposure to understand operational risk under different sourcing scenarios. Where possible, batch certificate of analysis trends and audit reports were examined to verify supplier quality claims and to identify common nonconformities that influence qualification risk. The research deliberately focused on observable practices and documented evidence rather than speculative market sizing, emphasizing actionable intelligence that supports immediate decision-making. Throughout the study, cross-validation between interview data and documented sources ensured that findings reflect both practitioner realities and technical constraints relevant to pharmaceutical-grade potassium chloride.
Concluding perspectives emphasizing the imperative to harmonize technical specifications supply diversification and governance to secure pharmaceutical product integrity
Ensuring consistent access to pharmaceutical-grade potassium chloride requires a blend of technical rigor, operational foresight, and strategic contracting. High-purity materials are central to the safety and efficacy of both injectable and oral electrolyte therapies, and suppliers must demonstrate validated analytical capabilities and transparent provenance. At the same time, the changing geopolitical and regulatory environment necessitates that manufacturers adopt multi-sourcing strategies, invest in traceability, and strengthen cross-functional governance to manage change without disrupting patient access.Decision-makers should prioritize interventions that reduce qualification time and supply risk while preserving product quality. These include insisting on harmonized testing protocols across suppliers, negotiating contractual protections for tariff and supply volatility, and leveraging distributors that provide pharmaceutical-grade services. By combining these tactical measures with longer-term investments in digital traceability and regional manufacturing resilience, organizations can protect their product pipelines and maintain regulatory compliance. Ultimately, the ability to align technical specifications with supply strategies will determine which organizations deliver consistent patient outcomes while navigating an increasingly complex sourcing environment.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- Amneal Pharmaceuticals, Inc.
- Aurobindo Pharma Limited
- Baxter International Inc.
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- Endo International plc
- Glenmark Pharmaceuticals Ltd.
- Hikma Pharmaceuticals PLC
- Hospira, Inc.
- ICU Medical, Inc.
- Intas Pharmaceuticals Ltd.
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Sandoz International GmbH
- Shanxi C&Y Pharmaceutical Group
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Wockhardt Ltd.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
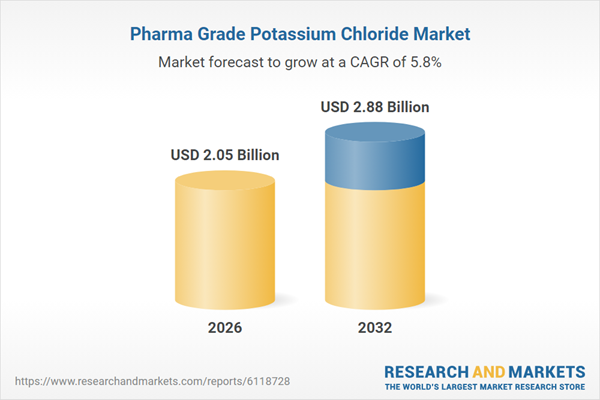
| Estimated Market Value ( USD | $ 2.05 Billion |
| Forecasted Market Value ( USD | $ 2.88 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |