Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introductory overview framing clinical, laboratory, regulatory, and commercial drivers that are reshaping oocyte collector adoption and supplier strategy
This executive introduction situates the medical oocyte collector domain at the intersection of clinical practice evolution, laboratory research expansion, and manufacturing innovation. Recent years have seen a convergence of technological advances in collection mechanics, materials science, and automation that have redefined operator workflows and product development priorities. Clinicians and laboratory managers are balancing competing priorities: improving patient outcomes through procedural efficiency and minimizing contamination risk while managing cost, disposables usage, and supply continuity. In parallel, research institutions and commercial organizations are pursuing specialized collection protocols to support genetic and stem cell research, creating distinct technical requirements that influence product design and procurement decisions.The introduction also highlights the growing importance of regulatory and quality frameworks that govern invasive reproductive procedures and laboratory consumables. Stakeholders are increasingly oriented toward traceability, biocompatibility certification, and validated sterilization pathways, which have implications for both single-use disposables and reusable devices. Moreover, distribution dynamics are shifting as manufacturers refine omnichannel strategies that combine direct engagements with clinics, distributor partnerships, and e-commerce touchpoints to support rapid replenishment and technical support. The balance between innovation, safety, and cost-effectiveness will continue to shape buyer behavior and supplier differentiation across clinical and research settings.
Finally, the introduction underscores the strategic imperative for organizations to integrate insights across clinical end users, product types, collection methods, and distribution channels. Understanding the nuanced needs of fertility clinics, hospital systems, and academic or commercial research laboratories is essential for designing solutions that are both clinically acceptable and operationally sustainable. This framing sets the stage for subsequent sections, which examine transformative shifts, tariff impacts, segmentation intelligence, regional dynamics, and practical recommendations for leaders navigating this evolving landscape.
Strategic transformation driven by automation, material innovations, and collaborative R&D partnerships altering the future of oocyte collection practice
The landscape of oocyte collection is undergoing transformative shifts driven by technological maturation, changing clinical protocols, and evolving stakeholder expectations. Automation and digitization are no longer niche discussions; motor-driven aspirators and robotic-assisted platforms are increasingly integrated into advanced fertility centers and research laboratories, reducing operator variability and improving procedural reproducibility. These trends are accompanied by parallel advances in imaging and sensor integration, which enable real-time monitoring of collection parameters and contribute to improved sample integrity. As a result, procurement teams and R&D units are prioritizing systems that deliver consistency while enabling data capture for quality assurance and regulatory documentation.Material science innovations are redefining product portfolios, with single-use plastics and silicone disposables offering convenience and contamination control while reusable glass and stainless-steel alternatives are being re-engineered for ease of sterilization and lifecycle longevity. Environmental sustainability considerations are prompting manufacturers to explore lower-carbon production processes, recyclable packaging, and designs that reduce resource intensity without compromising sterility or performance. Meanwhile, distribution models are shifting to accommodate a hybrid approach: manufacturers with direct sales capabilities are reinforcing clinical relationships, distributors are extending regional reach and after-sales support, and e-commerce platforms are providing rapid fulfillment pathways for standardized consumables.
Lastly, cross-sector collaboration is accelerating innovation. Academic research institutes and commercial research organizations are partnering with device manufacturers to validate novel collector geometries and collection protocols tailored to genetic and stem cell research. These collaborations shorten development cycles and create a pipeline of specialized products that address niche but high-value laboratory needs. Collectively, these transformative shifts are creating a bifurcated market dynamic in which high-volume clinical consumables and specialized research instruments follow distinct innovation and go-to-market trajectories, demanding differentiated strategies from suppliers and purchasers alike.
Analysis of how 2025 United States tariff adjustments are prompting supply chain realignment, sourcing diversification, and procurement risk mitigation across the value chain
The 2025 tariff environment in the United States has introduced a new layer of complexity across manufacturing, supply chain configuration, and procurement planning for oocyte collection devices and consumables. Import levies on certain medical components and raw materials have compelled manufacturers to reassess sourcing strategies, leading some to diversify supplier bases and to localize production of critical components to mitigate exposure. This reconfiguration has had cascading effects on lead times for both disposable and reusable collector lines and has intensified conversations about inventory management among clinical buyers and laboratory purchasers.Procurement teams in fertility clinics, hospitals, and research laboratories are responding by layering risk mitigation tactics into supplier contracts. These tactics include longer-term agreements with guaranteed supply windows, dual-sourcing arrangements that prioritize suppliers in tariff-exempt jurisdictions, and increased collaboration with distribution partners to create regional buffer stocks. For organizations operating in research environments, where experimental continuity is essential, the emphasis on supply reliability has become a primary procurement criterion alongside device compatibility and regulatory compliance. At the manufacturing level, firms are accelerating cost optimization efforts through production process efficiencies, material substitutions that meet biocompatibility requirements, and design changes that reduce complexity without sacrificing clinical utility.
The tariff-driven recalibration is also influencing channel strategies. Distributors and direct manufacturers are investing in regional warehousing and logistics capabilities to preserve service levels in the face of cross-border cost volatility. E-commerce platforms, particularly manufacturer-hosted storefronts and established platforms, are playing a role in preserving access to standardized consumables by streamlining ordering and fulfillment workflows. Overall, the cumulative impact of tariffs has reinforced the importance of supply chain resilience and strategic procurement planning, prompting stakeholders across the value chain to prioritize flexibility and localized support structures.
In-depth segmentation intelligence revealing divergent requirements across clinical, research, product, collection, and distribution dimensions to inform differentiated strategies
Segmentation intelligence reveals distinct demand drivers and technical requirements across end users, product types, collection methods, distribution channels, and applications, each of which should inform product design and go-to-market choices. When analysed by end user, the market is studied across fertility clinics, hospitals, and research laboratories, with research laboratories further differentiated between academic institutes and commercial research organizations. Fertility clinics typically prioritize ease of use, sterility assurance, and rapid replenishment, whereas hospitals emphasize compatibility with broader surgical and anesthetic workflows. Academic research institutes often require configurable systems for protocol development, while commercial research organizations seek validated, high-throughput solutions that align with GLP and commercial timelines.Product type segmentation highlights a bifurcation between disposable oocyte collectors and reusable oocyte collectors; the disposable category is further examined across single-use plastic and single-use silicone variants, while reusable options are assessed for glass and stainless-steel construction. Disposable single-use solutions are favored where contamination control and simplified logistics are paramount, whereas reusable glass and stainless-steel collectors are chosen for their durability and cost-per-use profile in settings with validated sterilization infrastructure. Collection method segmentation distinguishes automated collection and manual collection, with automated approaches further divided into motor-driven and robotic-assisted systems. Automated motor-driven systems deliver consistency and throughput improvements for routine clinical operations, while robotic-assisted platforms enable high-precision procedures and integration with imaging subsystems for specialized research applications.
Distribution channel dynamics are segmented into direct sales, distributors, and e-commerce, with e-commerce further parsed into manufacturer website and third-party platform routes. Direct sales remain valuable for high-touch clinical accounts needing technical training and clinical support, while distributors extend reach into regional hospital systems and smaller clinics. E-commerce channels provide rapid fulfillment for standardized disposable consumables and offer transparency in inventory and lead times. Finally, application segmentation differentiates clinical IVF and research use, with research being further subdivided into genetic research and stem cell research. Clinical IVF purchasers frequently emphasize regulatory traceability and operator ergonomics, whereas genetic and stem cell research applications demand protocol flexibility, sample integrity, and compatibility with downstream analytical workflows.
Regional dynamics shaping adoption, regulatory navigation, and distribution strategies across the Americas, EMEA, and Asia-Pacific markets
Regional dynamics materially influence adoption patterns, regulatory pathways, and procurement behaviors across markets. In the Americas, adoption is driven by a dense network of fertility clinics and a mature private-pay clinical ecosystem that values procedural efficiency and patient experience. Regulatory frameworks and reimbursement environments in this region shape buyer preferences toward devices and consumables that offer clear clinical utility and compliance documentation. Supply chains here emphasize speed to market and responsive technical support, prompting manufacturers to maintain local inventory and service resources to serve time-sensitive clinical schedules.Europe, the Middle East & Africa presents a heterogeneous landscape where regulatory regimes vary significantly across jurisdictions, and procurement channels include both centralized hospital tenders and private clinic networks. Clinical practice standards and infection control protocols in many EMEA markets place a premium on validated single-use options, while centers of excellence and research institutes in select countries continue to use reusable collectors under stringent sterilization protocols. Distribution in this region is frequently mediated by specialized distributors who provide local regulatory navigation, sterilization validation support, and technical training, making partnership selection a critical strategic consideration for manufacturers.
Asia-Pacific is characterized by rapid capacity expansion in both clinical IVF services and research infrastructure, fueled by demographic trends and investment in life sciences. High-volume fertility centers and an increasing number of commercial research organizations are adopting automated and robotic-assisted collection platforms, especially in markets prioritizing scalability and process standardization. In many Asia-Pacific markets, e-commerce penetration for standardized consumables is rising, enabling clinics and laboratories to access broad product assortments with improved lead times. Across regions, the interplay of regulatory requirements, infrastructure maturity, and procurement norms will continue to shape how suppliers prioritize investment and channel development.
Competitive and innovation landscape dominated by platform differentiation, regulatory readiness, and service models that drive supplier selection and partnership formation
Competitive dynamics among companies operating in the oocyte collector space are defined by technology differentiation, regulatory compliance, manufacturing scale, and channel depth. Leading suppliers are investing in platform architectures that support both disposable and reusable product lines, enabling them to address a broad spectrum of clinical and research use cases. Companies with established direct sales operations maintain close clinical relationships that inform iterative product improvements, whereas those leveraging distributor networks achieve broader geographic penetration and localized support for regulatory approvals and sterilization validation.Product innovation is concentrated in areas that improve clinical ergonomics, reduce procedure variability, and enhance sample integrity. This includes refined fluidics design, integrated sensors for aspiration pressure monitoring, and materials engineered for biocompatibility and sterilization resilience. In addition, service models that bundle training, validation protocols, and inventory management are emerging as differentiators, particularly for hospital systems and large fertility clinic networks. Strategic collaborations between manufacturers and research institutions accelerate the development of specialized collector designs tailored to genetic and stem cell workflows, enabling faster validation cycles and earlier market adoption in research niches.
Mergers, acquisitions, and distribution partnerships are reshaping competitive footprints, with mid-sized firms seeking alliances to expand technical capabilities or regional reach. At the same time, private and public funding for platform-level R&D continues to support entrants pursuing robotic-assisted solutions and advanced automation. For purchasers, supplier selection increasingly hinges on demonstrated quality systems, responsiveness, and the ability to deliver training and post-sale technical support that align with institutional protocols and regulatory expectations.
Actionable strategic recommendations for suppliers and purchasers to balance innovation, supply resilience, regulatory compliance, and service differentiation
Industry leaders should adopt a proactive, multi-dimensional strategy to capture clinical and research opportunities while managing supply chain risk and regulatory complexity. First, prioritize flexible product portfolios that combine validated single-use options with durable reusable systems designed for validated sterilization pathways to address the distinct needs of fertility clinics, hospitals, and research laboratories. This dual approach preserves clinical choice while enabling cost control and environmental stewardship initiatives.Second, invest in automation and data integration capabilities that reduce operator variability and provide auditable quality metrics. Motor-driven aspirators and modular robotic-assisted platforms should be designed with interoperability in mind, enabling seamless integration with imaging systems and laboratory information management systems. Third, strengthen supply chain resilience through supplier diversification, regional warehousing, and dual-sourcing strategies for critical materials. Close collaboration with distribution partners and selective investment in e-commerce channels will help preserve access to standardized consumables and reduce fulfillment lead times.
Fourth, build comprehensive service offerings that include clinical training, sterilization validation support, and post-sale technical assistance to differentiate in hospital and large-clinic accounts. Fifth, pursue targeted collaborations with academic institutes and commercial research organizations to co-develop solutions for genetic and stem cell workflows; these partnerships accelerate validation and create early-adopter references. Finally, establish clear environmental and regulatory positioning by documenting material biocompatibility, lifecycle analyses, and sterilization protocols to align with institutional procurement criteria and emerging sustainability expectations.
Robust mixed-methods research approach combining primary stakeholder interviews, peer-reviewed evidence, and supply chain analysis with iterative expert validation
The research methodology underpinning this analysis integrates primary qualitative engagement with secondary evidence and rigorous validation protocols to ensure reliability and relevance. Primary research included structured interviews with clinical leaders in fertility clinics and hospitals, device procurement managers, and laboratory directors in academic and commercial research organizations. These engagements explored operational priorities, device performance expectations, sterilization and quality assurance workflows, and procurement decision criteria. Interview inputs were triangulated to identify recurring themes and to calibrate the implications for product design and distribution strategy.Secondary research encompassed a systematic review of recent peer-reviewed literature on oocyte retrieval techniques, device sterilization standards, and materials science innovations, along with regulatory guidance documents relevant to medical device classification and laboratory consumable safety. Industry trade publications and conference proceedings were consulted to capture emerging automation and robotic-assisted developments. Supply chain analyses drew on customs and trade data where publicly available to assess import patterns and the likely impact of tariff measures on component sourcing.
Data synthesis involved cross-validation between primary interview insights and secondary sources, with particular attention to reconciling clinical practice variability across regions. Quality control steps included methodological transparency, interviewee anonymity to encourage candid responses, and iterative review cycles with subject-matter experts to confirm technical accuracy. Limitations are acknowledged where proprietary procurement data or recent tariff rulings may not be fully public, and recommendations are framed to be actionable within those bounds.
Concise conclusion synthesizing strategic imperatives, supply resilience priorities, and regional considerations that will determine success in the evolving oocyte collector sector
In conclusion, the oocyte collector landscape is being reshaped by concurrent forces: technological innovation in automation and materials, shifting distribution models, regulatory and sterilization requirements, and supply chain pressures exacerbated by tariff changes. These forces are creating distinct pathways for clinical consumables and specialized research instruments, requiring suppliers to adopt differentiated strategies that address the operational priorities of fertility clinics, hospitals, academic research institutes, and commercial research organizations. Strategic clarity around product portfolios, channel strategies, and service offerings will be essential to meet the divergent needs of these end users.Supply chain resilience and regulatory readiness have emerged as critical determinants of supplier credibility, particularly in an environment where import costs and lead-time volatility can interrupt clinical and research workflows. Investment in automation and data-enabled devices offers opportunities to enhance procedural consistency and to capture value through integrated services that support validation and operator training. Regional nuances in procurement practice and regulatory regimes mean that market entry and expansion strategies must be tailored rather than templated, with an emphasis on local partnerships and regulatory navigation support.
Ultimately, organizations that combine technological differentiation with strong service models, robust supply chains, and targeted regional strategies will be best positioned to support clinicians and researchers while sustaining commercial performance. The next sections provide practical steps for purchase and engagement to translate these insights into operational plans and procurement decisions.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- BPB Medica
- Cook Medical LLC
- CooperSurgical Inc.
- Corning Incorporated
- ESCO Micro Pte. Ltd.
- FUJIFILM Irvine Scientific Inc.
- Genea Biomedx Pty Ltd
- Gynemed Medizinprodukte GmbH & Co. KG
- Gynetics Medical Products
- Hamilton Thorne, Inc.
- IVFtech ApS
- Kitazato Corporation
- MedGyn Products, Inc.
- Merck KGaA
- Nidacon International AB
- Nipro Corporation
- Rocket Medical plc
- Smiths Medical
- Stryker Corporation
- Terumo Corporation
- Vitrolife AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
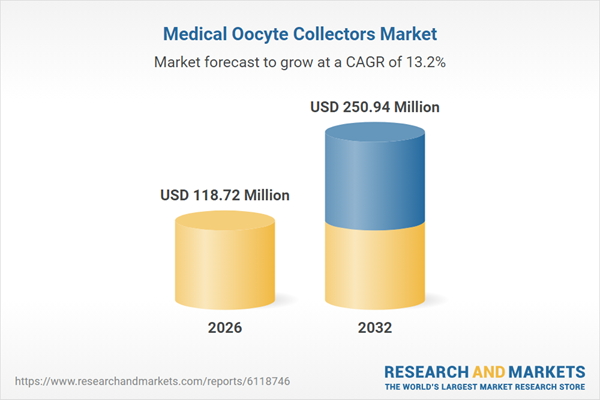
| Estimated Market Value ( USD | $ 118.72 Million |
| Forecasted Market Value ( USD | $ 250.94 Million |
| Compound Annual Growth Rate | 13.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |