Speak directly to the analyst to clarify any post sales queries you may have.
Concise but authoritative orientation to device attributes, clinical applicability, and procurement considerations for polypropylene hollow fiber hemodialyzers across the care continuum
Introduction to polypropylene hollow fiber hemodialyzers and their strategic role across contemporary renal therapy settings
Polypropylene hollow fiber hemodialyzers represent a distinct segment of extracorporeal blood purification devices characterized by polymer-based tubing and fiber architecture that supports efficient solute exchange while offering manufacturability advantages. Clinicians and procurement teams have increasingly evaluated these devices on criteria that include membrane compatibility with high- and low-flux modalities, sterilization pathways, disposability, and adaptability for in-center and home-based dialysis environments. Consequently, manufacturers and healthcare providers must reconcile clinical performance requirements with operational imperatives such as sterilization logistics, distribution footprints, and total cost of care.Across clinical settings the relative portability, ease of integration with emerging home dialysis modalities, and potential for standardized manufacturing processes have elevated the relevance of polypropylene designs. At the same time, regulatory scrutiny, material science innovations, and payer expectations have shaped product development priorities. These converging forces demand that stakeholders-from clinicians and biomedical engineers to supply chain managers and payers-understand how device attributes interact with care delivery models and procurement strategies. The introduction that follows establishes the analytical frame used throughout this report: clinical utility, material attributes, sterilization implications, usage patterns, and distribution dynamics, each examined through rigorous evidence synthesis and stakeholder engagement.
Emerging technological advances, care delivery decentralization, sterilization evolution, and sustainability drivers that are redefining competitive advantage in the hemodialyzer sector
Transformative shifts reshaping the polypropylene hollow fiber hemodialyzer landscape driven by technology, care models, and regulatory evolution
The last several years have witnessed a series of transformative shifts that are restructuring how polypropylene hollow fiber hemodialyzers are designed, delivered, and adopted. Advances in polymer processing and membrane engineering have improved flow dynamics and biocompatibility, enabling a clearer differentiation between high flux and low flux designs and influencing clinician preference across varied therapeutic protocols. Parallel to material innovations, sterilization technologies including ethylene oxide, gamma irradiation, and steam sterilization have evolved in terms of throughput, regulatory acceptance, and supply chain implications, prompting manufacturers to optimize production lines accordingly.Concurrently, care delivery models have pivoted toward greater decentralization: home healthcare programs that encompass assisted dwellings and self-care scenarios are expanding, while hospitals and standalone clinics evolve their service mixes between private, public, chain, and independent operations. These changes reinforce the need for product variants that meet diverse clinical workflows and sterilization logistics. In addition, sustainability imperatives and circular economy thinking are prompting manufacturers to re-evaluate disposable versus reusable usage models, material selection, and end-of-life handling. Regulatory frameworks and reimbursement paradigms are also shifting focus toward value-based outcomes, which incentivizes devices that align operational efficiency with measurable patient benefit. Taken together, these technological, clinical, and policy shifts are accelerating a redefinition of competitive advantage in the hemodialyzer sector.
Assessment of the multilayered effects of the 2025 United States tariff measures on procurement choices, supply chain resilience, and clinical continuity for hemodialysis devices
Cumulative implications of United States tariff changes in 2025 for procurement, supply chain resilience, and clinical access to polypropylene hollow fiber hemodialyzers
The adoption of tariff measures in 2025 introduced multi-dimensional pressures across procurement, manufacturing, and clinical operations for devices relying on cross-border supply chains. Increased duties on imported polymers, components, or finished devices raised the salience of sourcing strategy and supplier diversification for healthcare providers and manufacturers alike. Purchasing teams faced choices between absorbing higher landed costs, reallocating procurement to domestic or preferential trade partners, or accelerating localization investments to mitigate future trade volatility. Each pathway carried downstream effects on capital allocation, lead time expectations, and inventory management practices.In response, manufacturers examined options to reconfigure supply chains, including near-shoring production, qualifying alternate sterilization capacity within domestic jurisdictions, and revising commercial terms for direct sales and third-party distribution partners. Hospitals, chain clinics, and independent providers evaluated contract renegotiations and bulk procurement strategies to stabilize supply and protect clinical continuity. Importantly, tariff-related cost pressures intersected with regulator and payer scrutiny over pricing and value; this prompted procurement stakeholders to create more explicit evaluations of lifecycle costs, sterilization throughput, and compatibility with high- or low-flux clinical protocols. Ultimately, the 2025 tariff environment emphasized resilience and adaptability, compelling stakeholders to align sourcing, manufacturing investments, and clinical pathways to reduce vulnerability to geopolitical and trade perturbations.
Holistic segmentation analysis showing how end user needs, product flux types, sterilization choices, membrane sizing, usage models, and distribution channels determine product fit and adoption
Segmentation-focused insights that reveal demand drivers, clinical alignment, and operational implications across end users, product types, sterilization methods, membrane surface areas, usage models, and distribution channels
End user segmentation illustrates how demand and product requirements diverge between home healthcare scenarios, hospital dialysis centers, and standalone dialysis clinics. Home healthcare settings require devices compatible with assisted dwellings and self-care contexts, emphasizing ease of use, compactness, and sterilization methods that support lower-volume but higher-distribution complexity. Hospital dialysis centers-which encompass both private and public hospitals-prioritize throughput, regulatory compliance, and integration with institutional sterilization and waste streams. Standalone dialysis clinics, represented by chain clinics and independent clinics, balance cost-efficiency with standardized consumables to sustain high patient turnover while managing procurement centrally or locally.Product type distinctions between high flux and low flux remain clinically consequential, as high flux devices support enhanced middle molecule clearance for select patient populations while low flux devices may be preferred in other therapeutic settings or cost-sensitive programs. Sterilization method segmentation-ethyene oxide sterilized, gamma irradiated, and steam sterilized-shapes manufacturing cadence, logistics, and shelf-life considerations; for example, gamma irradiation can enable streamlined distribution to remote homecare channels, whereas steam sterilization may be more prevalent where centralized sterilization capacity exists. Membrane surface area choices across ranges greater than 2.0 square metres, 1.8 to 2.0 square metres, and less than 1.8 square metres influence device clearance characteristics and connector compatibility with varying dialysis machines. Product usage models-disposable versus reusable-drive procurement frequency, cleaning protocols, and environmental considerations. Finally, distribution channel structures, spanning direct sales and third-party distributors, determine sales strategy and customer service expectations; direct sales often leverage corporate sales and institutional contracts to engage hospitals and clinic networks, while third-party distributors-through dealers and online distributors-extend reach into geographically diverse home healthcare markets.
Regional strategic contrasts across the Americas, Europe Middle East & Africa, and Asia Pacific that drive differentiated procurement, product design, and distribution strategies
Regional dynamics and differentiated strategic priorities across the Americas, Europe Middle East & Africa, and Asia-Pacific that influence product design, procurement, and clinical adoption
In the Americas, decision-making centers around a mix of institutional contracting, regulatory harmonization, and commercial scale. Hospitals and large clinic chains often deploy centralized procurement teams that favor standardized disposable device models to streamline operations, while home healthcare growth steers interest toward devices that can be safely managed in assisted dwellings and self-care settings. Supply chain responsiveness and tariff exposure influence whether manufacturers maintain local production footprints or rely on near-shore suppliers to meet demand fluctuations.Across Europe, the Middle East, and Africa, heterogeneity in healthcare financing, regulatory frameworks, and infrastructure necessitates adaptable product portfolios. Public hospitals and private institutions may require different sterilization approaches and membrane configurations to satisfy national regulations and clinical protocols. In many jurisdictions, sustainability mandates and waste management constraints also shape preferences for reusable versus disposable options. The Asia-Pacific region exhibits rapid uptake of home-based modalities in some markets, combined with significant investment in manufacturing capacity and sterilization infrastructure in others. Local production capabilities, regulatory approvals, and distribution networks-especially third-party dealers and online channels-play decisive roles in shaping product availability across this region. Collectively, these regional variations underscore the importance of tailored commercial strategies and regulatory engagement for manufacturers and healthcare providers operating internationally.
Company-level strategic intelligence covering product differentiation, sterilization capacity, supply chain resilience, and distribution strategies that shape competitive positioning
Competitive and strategic company-level insights highlighting differentiation, operational priorities, and partnership strategies within the polypropylene hollow fiber hemodialyzer ecosystem
Leading companies in this sector differentiate through a combination of material science advancements, sterilization capability, and distribution architecture. Firms that invest in membrane engineering to support specific clinical profiles such as high flux clearance build clinical differentiation, while those that optimize sterilization throughput and logistics gain distribution advantages for home healthcare channels. Strategic partnerships with hospital systems, clinic chains, and homecare providers enable early clinical validation and facilitate pathway integration, particularly for products positioned for assisted dwellings and self-care use cases.Operationally, successful companies emphasize supply chain resilience, modular manufacturing lines that can accommodate ethylene oxide, gamma irradiation, and steam sterilization pathways, and flexible membrane surface area offerings that align with device compatibility across machines. Commercial strategies vary: some firms favor direct sales models supported by corporate sales and institutional contracts to secure long-term placements in hospitals and networks, while others expand reach through third-party distributors, dealers, and online channels to capture fragmented clinic and homecare demand. Firms pursuing mergers, acquisitions, or licensing arrangements often do so to broaden their sterilization capabilities, access new distribution networks, or accelerate time-to-market for differentiated membrane designs. Ultimately, sustained competitive advantage hinges on aligning clinical evidence, manufacturing agility, and distribution depth with evolving care delivery models.
Clear and executable strategic recommendations for manufacturers and providers to enhance manufacturing flexibility, clinical alignment, supply chain resilience, and commercial reach
Actionable recommendations for industry leaders to optimize clinical adoption, supply chain robustness, and commercial performance of polypropylene hollow fiber hemodialyzers
First, prioritize modular manufacturing investments that permit rapid switching between sterilization methods and membrane surface area configurations to serve hospital, clinic, and homecare segments without lengthy retooling. Such flexibility reduces lead-time risk and improves responsiveness to institutional contracting demands. Second, create dedicated product pathways for home healthcare use that account for assisted dwelling and self-care requirements; this includes simplified setup, robust instructions for end users, and sterilization approaches that support distributed logistics. Third, formalize procurement playbooks that account for tariff exposure and supply chain contingencies by maintaining multi-sourced polymer procurement, qualifying alternate sterilization partners, and exploring near-shore manufacturing options to reduce landed cost volatility.Fourth, engage clinical and reimbursement stakeholders early to align device attributes-such as high flux versus low flux performance and membrane surface area choices-with payer expectations and clinical protocols. Fifth, diversify distribution channels by blending direct sales supported through corporate sales and institutional contracts with selective third-party distributor relationships that extend geographic reach into remote or fragmented homecare markets. Sixth, embed sustainability and lifecycle management into product design decisions to meet regulatory expectations and institutional environmental goals. Finally, invest in post-market surveillance and outcomes measurement to generate real-world evidence that supports clinical differentiation and informs iterative product development.
Comprehensive and transparent research methodology integrating stakeholder interviews, technical specification review, regulatory analysis, and multi-source triangulation to validate findings
Research methodology and validation approach combining primary stakeholder engagement, device specification review, regulatory analysis, and triangulation to ensure robust market insights
This analysis synthesizes multiple evidence streams to produce balanced, actionable insights. Primary research included structured consultations with clinicians, procurement leaders, biomedical engineers, and distribution partners to capture operational realities across hospitals, chain clinics, independent clinics, and home healthcare environments. Secondary sources comprised publicly available regulatory filings, clinical literature, device specifications, and trade publications to map technology attributes such as membrane surface area, flux characteristics, and sterilization modalities. These inputs were triangulated to reconcile differences between clinical priorities and procurement constraints.Quality assurance measures included cross-validation of technical specifications with manufacturer disclosures, review of sterilization process capabilities against regulatory guidance, and scenario analysis to explore how tariff shifts and distribution choices influence operational practice. Limitations were explicitly acknowledged, including variability in national regulatory processes and the evolving nature of homecare adoption. Where possible, findings were corroborated across multiple stakeholder types to improve reliability. Confidentiality and ethical research standards were observed in all primary engagements to protect proprietary information and ensure candid stakeholder feedback.
Strategic synthesis of clinical requirements, operational constraints, and commercial levers that executives should use to navigate the evolving hemodialyzer landscape
Conclusion synthesizing clinical priorities, operational imperatives, and strategic levers for decision makers in the polypropylene hollow fiber hemodialyzer domain
Polypropylene hollow fiber hemodialyzers occupy a strategic intersection of material innovation, sterilization logistics, and care delivery transformation. Clinical decision making differentiates devices by flux performance and membrane surface area requirements, while operational considerations such as sterilization methods, disposable versus reusable usage, and distribution channels determine real-world deployability. The contemporary environment-shaped by home healthcare growth, regulatory scrutiny, regional variations, and tariff-induced supply chain pressures-favors organizations that can align product development with flexible manufacturing, evidence generation, and diversified distribution.For stakeholders across hospitals, clinics, and homecare programs, the imperative is to integrate clinical efficacy with procurement resilience. Manufacturers must enhance their capacity to offer targeted device variants, support multiple sterilization pathways, and establish distribution models that balance direct institutional relationships with third-party reach. For provider organizations, the focus should be on aligning procurement, clinical protocols, and training to ensure safe and effective use across both centralized and decentralized settings. By prioritizing adaptability, evidence-based differentiation, and collaborative partnerships, stakeholders can better navigate the evolving landscape and optimize patient outcomes.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China PP Hollow Fiber Hemodialyzer Market
Companies Mentioned
- Asahi Kasei Medical Co., Ltd.
- B. Braun Melsungen AG
- Baxter International Inc.
- Edges Medicare Pvt. Ltd.
- Fresenius Medical Care AG & Co. KGaA
- Guangdong Biolight Meditech
- Hemoclean Meditech Private Limited
- Medica S.p.A.
- Nikkiso Co., Ltd.
- Nipro Corporation
- Okamoto Medical Instruments Co., Ltd.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- SWS Hemodialysis Care Co., Ltd.
- Toray Industries, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
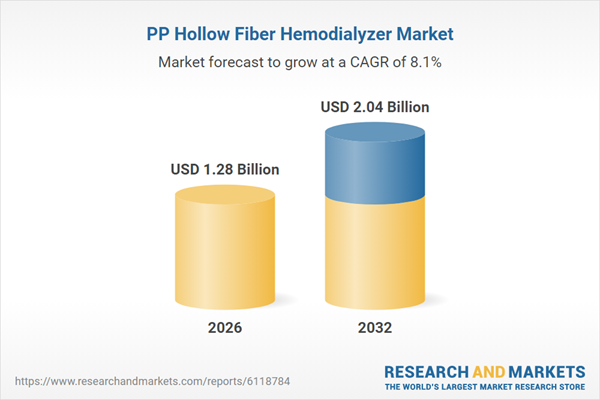
| Estimated Market Value ( USD | $ 1.28 Billion |
| Forecasted Market Value ( USD | $ 2.04 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |