Speak directly to the analyst to clarify any post sales queries you may have.
A concise, authoritative introduction summarizing the clinical rationale, strategic implications, and cross-specialty relevance of complement protein C3 inhibition for executive decision-makers
Complement protein C3 inhibition has moved from a niche scientific pursuit to a pivotal therapeutic approach with cross-disciplinary relevance in ophthalmology, nephrology, hematology, and autoimmune disease management. Advances in molecular biology and translational immunology have clarified C3's central role in complement cascade activation, driving renewed clinical interest and platform diversification. Consequently, stakeholders across biopharma, clinical practice, and specialty distribution are re-evaluating pipelines and partnerships to capture the therapeutic and procedural implications of next-generation C3 inhibitors.The contemporary landscape is characterized by a widening spectrum of indications under active investigation, including degenerative retinal conditions, thrombotic microangiopathies, autoimmune renal disorders, and complement-mediated hematologic diseases. These clinical targets differ markedly in patient demographics, treatment duration expectations, and outcome measures, creating a complex development and commercialization landscape. As such, strategic planning must balance scientific rigor with operational feasibility, particularly when transitioning from intravenous to more patient-friendly subcutaneous or oral modalities.
Given these dynamics, executives should expect sustained innovation coupled with intensifying scrutiny from regulators and payers. Integration of robust biomarker strategies, adaptive trial designs, and patient-centric delivery formats will be essential. The subsequent sections of this executive summary synthesize transformative shifts, tariff-related implications, segmentation insights, regional differentials, competitive positioning, recommended actions, methodological transparency, and a succinct conclusion to inform high-stakes decision-making.
How recent scientific breakthroughs, regulatory flexibility, and shifting commercialization priorities are reshaping development choices and patient-centric delivery strategies for C3 inhibitors
The competitive and clinical landscape for C3 inhibitors is undergoing transformative shifts driven by converging scientific, regulatory, and commercial forces. Scientific advances in complement biology have deepened understanding of C3's upstream role, enabling therapeutic concepts that aim for broader pathway modulation rather than isolated terminal blockade. This conceptual shift has prompted firms to expand candidate profiles across monoclonal antibodies, peptides, and small molecules to balance potency, specificity, and manufacturability.Concurrently, development strategies are shifting toward patient-centric administration routes and pragmatic trial designs. There is clear momentum toward subcutaneous and oral modalities to reduce hospital dependency and improve adherence, while intravenous formulations remain critical for acute and inpatient settings. Regulatory agencies are increasingly receptive to adaptive and biomarker-driven trials that can accelerate proof-of-concept while preserving evidentiary rigor. In parallel, real-world evidence initiatives and post-authorization safety studies are becoming integral to reimbursement narratives and label expansion efforts.
Commercially, partnerships and licensing deals are extending beyond traditional alliances to include technology providers for delivery devices, specialty pharmacy networks for distribution, and digital therapeutics vendors for adherence solutions. As a result, organizations must reconcile long-range scientific ambition with near-term operational realities, optimizing development portfolios to balance high-potential but riskier assets with those that offer incremental clinical and commercial value.
Assessment of the operational and supply chain consequences triggered by the 2025 tariff landscape and their implications for sourcing, clinical logistics, and commercial preparedness
The imposition of tariffs and related trade measures in 2025 introduced a layer of complexity for global supply chains and cost structures in biologics and small-molecule therapeutics, and C3 inhibitor programs are no exception. Increased tariffs on active pharmaceutical ingredients, biologics components, or specialized devices elevated landed costs and amplified the importance of supply chain resiliency. Manufacturers responded by reassessing sourcing strategies, repatriating critical manufacturing steps, or qualifying additional contract manufacturing organizations across diversified geographies.These adjustments had immediate implications for procurement timelines, inventory planning, and capital allocation for capacity expansions. Sponsors prioritized supplier audits and dual-sourcing arrangements to mitigate disruption risk while instituting more conservative inventory buffers during pivotal clinical phases. In parallel, clinical operations teams contemplated site selection and investigational product logistics with tariff exposure in mind, favoring regions or distribution pathways with lower trade friction where possible.
Policy instability also intensified payer and procurement scrutiny, prompting earlier engagement with health systems to discuss total cost of care and value-based contracting mechanisms. In summary, the tariff environment reinforced the strategic importance of flexible manufacturing footprints, regional supply redundancy, and commercially minded development decisions that anticipate evolving trade and procurement landscapes.
Detailed segmentation-driven insights combining indication, product type, administration route, distribution channel, and end-user dynamics to inform development and go-to-market priorities
Segmenting the C3 inhibitors landscape across indication, product type, route of administration, distribution channel, and end user yields actionable clarity about distinct development pathways and commercial routes to patients. When analyzed by indication, programs targeting age-related macular degeneration demand specialized ophthalmic endpoints, intravitreal or systemic delivery considerations, and close collaboration with retina specialists, whereas therapeutic approaches for atypical hemolytic uremic syndrome (AHUS) and paroxysmal nocturnal hemoglobinuria (PNH) must address hematologic markers, thrombosis risk, and long-term safety monitoring; lupus nephritis programs require integration with nephrology care pathways, immune modulation strategies, and renal outcome measures.Product type segmentation highlights trade-offs between platform characteristics: monoclonal antibodies offer high specificity and proven biologic pathways but often carry higher manufacturing complexity; peptides can provide intermediate profiles that balance target engagement with improved tissue penetration; small molecules present opportunities for oral dosing, cost-effective manufacturing, and potentially broader patient access. Route of administration further refines product strategy, with intravenous options remaining central for hospital-administered acute care and continuous dosing paradigms, while oral formulations in capsules, solutions, or tablets present clear advantages for chronic outpatient therapy. Subcutaneous delivery via auto-injectors, prefilled syringes, or prefilled pens aligns with home administration models and can materially influence adherence and patient preference.
Distribution channel and end user segmentation complete the picture by defining how therapies reach patients and who administers them. Clinics and hospital pharmacies underpin acute and specialty care settings, online and retail pharmacies expand outpatient accessibility and convenience, and home healthcare and specialty clinics play pivotal roles in long-term management and monitoring. Research institutes remain important early adopters and collaborators for translational studies. Taken together, these segmentation lenses guide prioritization of clinical endpoints, formulation choices, device partnerships, and channel strategies that together determine how therapies are developed and scaled to meet clinical needs.
Comprehensive regional intelligence outlining regulatory, clinical infrastructure, reimbursement, and manufacturing considerations across the Americas, EMEA, and Asia-Pacific to guide strategic planning
Regional dynamics significantly affect clinical development, regulatory engagement, manufacturing footprint decisions, reimbursement pathways, and commercial rollout strategies. In the Americas, established clinical research infrastructure, concentrated specialty care networks, and evolving payer models create fertile ground for early launch activities and evidence generation, while regulatory interactions demand clear benefit-risk communication and robust post-authorization safety planning. Latin American sub-regions present heterogeneous reimbursement environments but increasing interest in innovative therapies, necessitating tailored access strategies and localized health economic narratives.Europe, the Middle East & Africa present a mosaic of regulatory frameworks and payer decision-making paradigms where centralized and national level pathways interact. European markets often emphasize health technology assessment and comparative effectiveness, prompting sponsors to generate outcomes data and real-world evidence to support value arguments. Patient access can vary widely across countries, and device and delivery device acceptance can influence uptake. In the Middle East and Africa, constrained specialist capacity and fragmented procurement marketplaces require focused partnership strategies and targeted pilot implementations to demonstrate feasibility.
Asia-Pacific encompasses markets with rapid clinical research maturation, diverse regulatory timelines, and strong manufacturing capabilities. Several countries in the region are investing in clinical trial infrastructure and expedited review pathways for innovative therapies. Local manufacturing and regional hubs can reduce tariff exposure and shorten supply chains, while reimbursement negotiation norms differ significantly across jurisdictions, calling for adaptive pricing and access strategies. Across all regions, cross-border data sharing, digital engagement, and patient-centric delivery innovations are accelerating, underscoring the importance of regionally nuanced commercialization blueprints.
Key competitive positioning and capability insights highlighting differentiators in development platforms, device partnerships, biomarker strategies, and manufacturing resilience across the industry
Competitive dynamics among established biopharmaceutical firms, emerging biotechnology companies, and specialty device partners are redefining how C3 inhibitor candidates advance from discovery to clinical application. Companies with integrated capabilities in biologics development and scalable manufacturing maintain advantages in monoclonal antibody programs, while nimble biotechnology firms are leveraging platform technologies and targeted chemistry to advance peptide and small-molecule portfolios. Strategic partnerships with device manufacturers and specialty pharmacy providers are increasingly common as developers seek to optimize route-of-administration and patient support services.Investment in biomarker science and companion diagnostic development differentiates leading organizations, enabling targeted patient selection and adaptive trial designs. Firms that combine clinical expertise with commercial channel know-how - including hospital systems, specialty clinics, and digital health partners - tend to accelerate uptake post-approval. Additionally, companies that prioritize manufacturing resilience through multi-site capacity, robust quality systems, and flexible fill-finish options are better positioned to respond to trade disruptions and scale rapidly when clinical programs succeed.
In this competitive environment, intellectual property strategy, regulatory experience, and demonstrated safety profiles form the core differentiators. Organizations that adopt agile development practices, maintain transparent regulatory dialogue, and invest in payer-centered evidence generation are most likely to convert clinical promise into durable clinical use and provider adoption.
Actionable, phased recommendations for leaders to align scientific priorities with operational resilience, regulatory strategy, and commercially viable delivery and access models
Industry leaders should adopt a phased, evidence-driven approach that aligns scientific ambition with operational practicality and commercial foresight. First, prioritize indications where clinical endpoints, patient populations, and delivery modalities align to create clear value propositions for clinicians and payers. Next, diversify product portfolios across monoclonal antibodies, peptides, and small molecules to hedge scientific and manufacturing risk while accelerating learning across modalities. Simultaneously, invest in subcutaneous and oral development pathways to capture outpatient market opportunities and enhance patient convenience.Operationally, build robust supply chain redundancy and flexible manufacturing arrangements that can adapt to tariff fluctuations and regional disruptions. Establish dual sourcing for critical biologic inputs and qualify multiple contract manufacturers to shorten response times. From a clinical perspective, embed biomarker strategies and adaptive trial elements early to refine patient selection and accelerate proof-of-concept. Engage regulatory authorities proactively to clarify evidentiary expectations and leverage accelerated pathways where clinically appropriate.
Commercially, form partnerships with device companies, specialty pharmacies, and digital health providers to enhance adherence, reduce administration burden, and strengthen real-world evidence collection. Develop reimbursement dossiers that anticipate payer concerns about long-term outcomes and total cost of care, and consider value-based contracting pilots to align incentives. Finally, invest in cross-functional capabilities that link clinical development with market access, manufacturing, and commercial deployment to ensure that strategic choices are executable across the organization.
Transparent explanation of the mixed-methods research approach combining expert interviews, literature synthesis, regulatory review, and scenario analysis to underpin strategic recommendations and insights
The research approach underpinning these insights combined multi-source synthesis, expert interviews, and scenario analysis to ensure a balanced and objective perspective. Primary inputs included structured interviews with clinical investigators, regulatory specialists, manufacturing experts, and commercial leaders involved in complement therapeutics. These dialogues provided qualitative context around clinical practice, trial design preferences, manufacturing constraints, and payer expectations.Secondary sources encompassed peer-reviewed literature, regulatory guidance documents, clinical trial registries, and public filings that informed the mechanistic rationale, therapeutic development status, and regulatory precedents for C3-targeted approaches. Additionally, supply chain and tariff policy analyses were incorporated to assess operational vulnerabilities and mitigation strategies. Scenario modeling was used to stress-test strategic options across variations in clinical trial outcomes, regulatory timelines, and trade environments, enabling pragmatic recommendations that account for uncertainty.
Throughout the methodology, assumptions and evidence were triangulated to minimize bias and ensure recommendations remain actionable across a range of plausible futures. This mixed-methods approach delivers a robust foundation for both strategic decision-making and operational planning in the evolving C3 inhibitor landscape.
Concise conclusion synthesizing the strategic imperatives and operational priorities necessary to realize clinical and commercial potential for complement protein C3 inhibitors
In conclusion, complement protein C3 inhibitors represent a strategically significant therapeutic frontier with broad application across ophthalmology, nephrology, hematology, and autoimmune disease management. Scientific advancements, evolving regulatory attitudes, and patient-centric delivery innovations are collectively driving development and commercialization opportunities. However, organizations must navigate complex operational challenges, including supply chain fragility, tariff exposure, and diversified reimbursement environments, to translate clinical potential into real-world impact.A deliberate strategy that aligns indication prioritization, product-type diversification, and route-of-administration optimization with robust manufacturing and distribution planning will be essential. Early and sustained engagement with regulators, payers, clinicians, and device partners can de-risk development pathways and accelerate adoption. Ultimately, success will favor organizations that combine rigorous biomarker-driven clinical programs with flexible operational models and targeted commercialization partnerships.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- AbbVie Inc.
- Alexion Pharmaceuticals, Inc.
- Amgen Inc.
- Apellis Pharmaceuticals, Inc.
- AstraZeneca PLC
- Biogen Inc.
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Catalyst Biosciences, Inc.
- Eli Lilly and Company
- Horizon Therapeutics plc
- Ionis Pharmaceuticals, Inc.
- Janssen Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Roche Holding AG
- Sanofi
- UCB S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
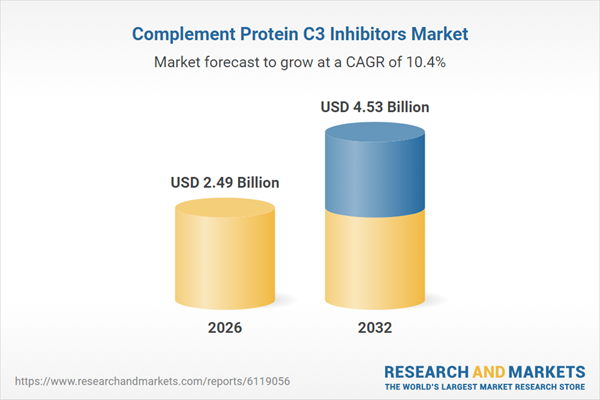
| Estimated Market Value ( USD | $ 2.49 Billion |
| Forecasted Market Value ( USD | $ 4.53 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |