Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive orientation to oral CDK4/6 inhibitors that frames clinical evolution, commercial pressures, and strategic priorities for stakeholders across the ecosystem
Oral CDK4/6 inhibitors have emerged as a cornerstone of targeted oncology therapeutics, fundamentally altering treatment paradigms for hormone receptor-positive malignancies and beyond. Over the past decade, these agents have transitioned from novel mechanism-of-action candidates to established components of multidisciplinary care, prompting shifts in trial design, biomarker strategies, and payer dialogues. This introduction outlines the clinical rationale, therapeutic differentiation, and strategic considerations that are essential for stakeholders who must navigate a progressively complex environment where efficacy, tolerability, and real-world performance determine adoption.The clinical narrative is anchored in a growing evidence base that supports combination approaches alongside endocrine therapy and a maturing safety profile that informs long-term management. As a result, clinicians, payers, and manufacturers are recalibrating expectations around sequencing, patient selection, and survivorship care. Concurrently, regulatory frameworks have adapted to accommodate accelerated pathways and label expansions driven by robust benefit-risk assessments, creating both opportunities and obligations for post-market evidence generation.
From a commercial perspective, the competitive interplay among differentiated molecules influences promotional strategies, procurement negotiations, and contracting models. Supply chain resilience and distribution optimization are increasingly integral to ensuring patient access, particularly where specialty channels bear responsibility for complex therapy management. In sum, this introduction frames oral CDK4/6 inhibitors as a dynamic therapeutic class at the intersection of clinical innovation and strategic market execution, setting the stage for the deeper analysis that follows.
How recent scientific breakthroughs, regulatory evolution, and payer focus on real-world outcomes are collectively transforming the competitive and clinical landscape for oral therapies
The oral CDK4/6 inhibitor landscape is undergoing transformative shifts driven by rapid scientific advances, evolving regulatory expectations, and heightened emphasis on personalized therapy pathways. Recent translational research has sharpened biomarker-driven approaches, enabling more precise patient stratification and sparking a wave of adaptive trial designs that aim to validate novel combination regimens and sequencing strategies. These scientific developments are catalyzing changes in clinical practice, as oncologists integrate genomic and phenotypic data to tailor treatment duration and manage toxicities proactively.Meanwhile, regulatory agencies have signaled greater flexibility for label modifications supported by real-world evidence and pragmatic clinical trial data, which in turn has encouraged sponsors to invest in longitudinal observational studies and registries. This shift reduces the latency between evidence generation and clinical adoption, but it also places a premium on robust pharmacovigilance infrastructures and transparent outcomes reporting. Payers and health technology assessment bodies are responding by refining value frameworks that emphasize comparative effectiveness, quality-of-life metrics, and total cost of care, prompting manufacturers to develop evidence dossiers that extend beyond traditional endpoints.
Commercially, competition among therapeutically similar agents has driven innovation in patient support services, pricing models tied to outcomes, and specialty distribution strategies that improve adherence and monitoring. At the same time, cross-sector collaboration-spanning diagnostics developers, advocacy groups, and integrated care providers-is redefining how access initiatives are designed and evaluated. Taken together, these factors are shifting the landscape from product-centric competition toward ecosystem-level differentiation, where service delivery, evidence generation, and stakeholder alignment determine sustained success.
Assessing the operational and strategic consequences of United States tariff changes in 2025 on supply resilience, procurement strategies, payer engagement, and patient access for oral therapies
Tariff policy shifts in the United States during 2025 introduced another layer of complexity for the supply chains and procurement strategies that underpin access to oral CDK4/6 therapies. These policy changes affected upstream inputs, packaging, and distribution costs, prompting stakeholders to reassess supplier diversification, manufacturing footprints, and inventory strategies to preserve margins and ensure continuous patient supply. Manufacturers responded by accelerating nearshoring and dual-sourcing initiatives to mitigate exposure to cross-border cost volatility, while distribution partners increased collaboration with contract manufacturers to maintain throughput under new cost constraints.In parallel, hospitals and specialty pharmacies adapted procurement protocols to balance cost pressures with clinical imperative, often negotiating longer-term agreements or capitated arrangements to stabilize unit pricing. Payers considered adjustments to formulary placement and prior-authorization criteria in light of the shifting cost basis, which in some instances led to more stringent utilization management while encouraging value-based contracting for high-cost therapies. Additionally, the tariff environment prompted an expanded focus on packaging optimization and consolidated shipments to reduce per-unit logistics expenses, with technology-enabled tracking used to protect cold chain integrity and expedite customs clearance.
Despite these operational headwinds, strategic responses have emerged that preserve patient access by aligning commercial incentives with clinical outcomes and supply resilience. Stakeholders that invested early in alternative sourcing strategies and transparent stakeholder communication found it easier to absorb tariff-related shocks, maintain continuity of care, and uphold commitments to adherence programs that are critical for long-term therapeutic effectiveness.
Deep segmentation insights that connect product attributes, therapeutic sequencing, regimen choices, distribution channels, and end-user capabilities to optimize adoption and access strategies
A fine-grained segmentation lens reveals how distinct product profiles, lines of therapy, therapy regimens, distribution channels, and end user dynamics shape commercialization and clinical adoption. Based on Product, the market perspective accounts for Abemaciclib, Palbociclib, and Ribociclib, each presenting unique tolerability, dosing, and monitoring considerations that influence guideline positioning and clinician preference. Based on Line Of Therapy, differentiation emerges between First Line approaches and Second Line Or Later settings, driving divergent evidence needs, patient selection criteria, and payer negotiation points. Based on Therapy Regimen, the interplay between Combination Therapy and Monotherapy dictates companion diagnostic strategies, safety surveillance burdens, and adherence support requirements that are distinct for each regimen type.Furthermore, Based on Distribution Channel, the distribution landscape spans Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy channels, each with separate logistical, reimbursement, and patient engagement implications that must be reconciled to ensure continuity of therapy. Based on End User, clinics, hospitals, and specialty pharmacies demonstrate varying operational competencies and care pathways, influencing how treatments are administered, monitored, and supported in the community. These segmentation dimensions interact to form complex adoption matrices: a product’s clinical attributes can favor a particular line of therapy, which in turn may be better served by specific distribution channels and end-user capabilities.
As a result, strategic planning must integrate segmentation insights across these axes to align clinical development priorities, commercialization playbooks, and access strategies. Tailored evidence generation, channel-specific patient support programs, and end-user-focused training can meaningfully enhance uptake while preserving the integrity of therapeutic outcomes across heterogeneous care settings.
Comparative regional analysis revealing how Americas, Europe Middle East & Africa, and Asia-Pacific dynamics uniquely influence access, reimbursement, and adoption pathways for therapies
Regional dynamics exert a profound influence on the adoption, reimbursement, and delivery of oral CDK4/6 therapies, with each geography presenting distinct regulatory regimes, payer architectures, and care delivery models. In the Americas, the interaction between private payers, public programs, and integrated delivery networks shapes formulary decisions and access pathways, with stakeholder negotiations often emphasizing rapid evidence translation and innovative contracting. In Europe, Middle East & Africa, heterogeneity across national health systems necessitates localized market access approaches that account for centralized assessments in some countries and decentralized evaluation in others, while emerging markets within the region present unique capacity constraints and affordability considerations. In Asia-Pacific, rapid regulatory modernization coexists with diverse reimbursement environments and varying healthcare infrastructure maturity, driving a mix of accelerated approvals in some markets and phased rollouts in others.These regional patterns affect not only how clinical data are generated and presented, but also how manufacturers structure distribution agreements, patient support services, and pricing models. For example, regions with strong centralized procurement may prioritize single-source supply agreements and volume-based pricing, whereas markets with a high proportion of private payers may emphasize tailored access programs and co-pay assistance. Cross-region differences in diagnostic availability and oncology workforce capacity also inform the feasibility of complex combination regimens and intensive monitoring protocols.
Consequently, successful regional strategies balance standardized global messages about clinical value with nimble, localized execution that addresses regulatory nuance, infrastructure constraints, and payer expectations. This approach enables more consistent patient access while optimizing commercial efficiency across diverse healthcare landscapes.
Company-level competitive intelligence demonstrating how clinical differentiation, service innovation, and strategic partnerships define competitive advantage and long-term positioning
Competitive dynamics among companies operating in the oral CDK4/6 space are shaped by differentiated clinical profiles, lifecycle management strategies, and complementary service offerings that extend beyond the molecule itself. Leading developers have pursued strategies that blend label expansions, combination trials with targeted agents, and investment in real-world evidence to sustain therapeutic leadership. Some companies concentrate on optimizing safety and tolerability to improve long-term adherence, while others prioritize broad population indications supported by large-scale pragmatic studies. Across the competitive set, partnerships with diagnostic firms and academic consortia have become pivotal to advancing precision medicine approaches and to accelerating patient identification in routine practice.Commercially, firms innovate in patient support by integrating telehealth monitoring, nurse-led adherence programs, and digital symptom-tracking tools that reduce drop-off and enhance persistence. These services have become differentiators in payer negotiations, where evidence of improved outcomes and reduced downstream resource utilization can offset higher upfront therapy costs. Additionally, strategic alliances with specialty pharmacy networks and hospital systems have enabled more reliable distribution and data capture, feeding back into post-market evidence generation.
Corporate development activity-including licensing, co-development agreements, and targeted acquisitions-continues to refine pipelines and expand therapeutic ecosystems. Organizations that align clinical differentiation with robust service capabilities and data-driven value propositions are positioned to secure durable relationships with clinicians, payers, and health systems, thereby strengthening their competitive moats in this evolving therapeutic category.
High-impact, actionable recommendations for industry leaders to align evidence generation, commercialization, supply resilience, and payer engagement for sustainable success
To succeed in an increasingly complex therapeutic environment, industry leaders should adopt a set of actionable strategies that integrate clinical, commercial, and operational priorities. First, prioritize evidence generation that addresses payer and clinician questions beyond traditional endpoints, including comparative effectiveness, health-related quality of life, and real-world safety signals; this will facilitate differentiated value communication across multiple stakeholder audiences. Second, invest in integrated patient support ecosystems that combine digital monitoring, adherence counseling, and streamlined pharmacy access to reduce drop-off and improve outcomes, thereby strengthening reimbursement negotiations and provider trust.Third, diversify manufacturing and sourcing footprints to mitigate exposure to geopolitical and tariff-related disruptions while building supply flexibility through dual sourcing and strategic inventory buffers. Fourth, align pricing and contracting models with measurable outcomes by piloting risk-sharing arrangements and indication-based pricing where feasible, thereby aligning commercial incentives with payer expectations. Fifth, pursue targeted regional strategies that respect local regulatory pathways and healthcare infrastructure, deploying adaptive evidence packages and localized payer engagement plans to accelerate uptake.
Finally, cultivate partnerships across diagnostics, academic centers, and patient advocacy groups to accelerate patient identification, enhance trial recruitment, and broaden real-world data collection. By operationalizing these recommendations in coordinated roadmaps, leaders can translate clinical promise into sustained patient access, payer acceptance, and commercial viability within the oral CDK4/6 therapeutic domain.
Transparent and reproducible research methodology articulating data sources, expert validation processes, analytical frameworks, and known limitations to support confident decision-making
The research underpinning this analysis relied on a multi-method approach designed to ensure analytical rigor, triangulation of evidence, and clarity around limitations. Primary inputs included expert interviews with oncologists, pharmacy directors, and market access specialists, which informed interpretation of clinical practice trends and operational constraints. Secondary sources comprised peer-reviewed literature, regulatory communications, and publicly available clinical trial registries to map evolving evidence and label changes. These data streams were synthesized using a structured analytical framework that emphasizes clinical differentiation, channel dynamics, and stakeholder incentives.Qualitative insights were validated through cross-stakeholder review sessions and scenario testing to assess the robustness of strategic implications under varying operational conditions. Where appropriate, sensitivity analyses were conducted to probe the impact of supply chain disruptions, regulatory shifts, and reimbursement policy changes on access pathways. The methodology prioritized transparency by documenting data provenance, assumptions, and areas of uncertainty, acknowledging that rapidly evolving clinical evidence and policy developments require ongoing monitoring.
Limitations of the research include the potential for rapid post-publication changes in clinical trial outcomes, regulatory decisions, or policy developments that could affect specific recommendations. To mitigate this, the research design incorporated iterative expert feedback loops and recommended mechanisms for continuous evidence surveillance to ensure that strategic actions remain aligned with the most current information.
A conclusive synthesis of strategic imperatives and clinical takeaways designed to guide decision-makers in translating therapeutic potential into durable patient access and commercial success
In conclusion, oral CDK4/6 inhibitors represent a matured therapeutic class whose future trajectory will be determined as much by strategic commercialization and evidence generation as by clinical innovation. The current environment rewards stakeholders who can integrate high-quality clinical data with pragmatic real-world evidence, deploy resilient supply and distribution strategies, and construct payer-aligned value propositions that are responsive to regional nuances. Coordination across diagnostic pathways, treatment settings, and patient support mechanisms will be essential to maximizing therapeutic benefit and ensuring equitable access across heterogeneous health systems.Looking ahead, the most successful organizations will blend disciplined clinical development with service-oriented commercialization, invest in adaptive sourcing and manufacturing models, and engage payers early with transparent, outcome-focused evidence. By doing so, they will reduce time-to-adoption, improve adherence and persistence, and strengthen payer relationships through demonstrable value. The strategic choices made today-about where to invest in data generation, how to structure distribution, and which partnerships to pursue-will shape not only competitive outcomes but also patient-level impacts for years to come.
Stakeholders should therefore approach the oral CDK4/6 landscape with a balanced emphasis on scientific rigor, operational resilience, and collaborative engagement to convert therapeutic potential into sustainable clinical and commercial success.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- Cipla Limited
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- Fosun Pharma (Shanghai)
- G1 Therapeutics, Inc.
- Genor BioPharma Co., Ltd.
- Gilead Sciences, Inc.
- Incyte Corporation
- Jiangsu Hengrui Pharmaceuticals Co., Ltd.
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
- Prelude Therapeutics, Inc.
- Sandoz International GmbH
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
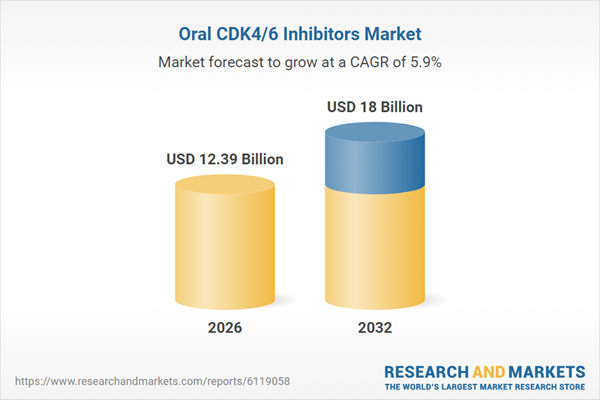
| Estimated Market Value ( USD | $ 12.39 Billion |
| Forecasted Market Value ( USD | $ 18 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |