Speak directly to the analyst to clarify any post sales queries you may have.
An incisive overview of absorbable fixation systems for hernia repair highlighting clinical rationale, product evolution, regulatory dynamics, and stakeholder implications
Absorbable fixation systems are increasingly central to contemporary hernia repair practice, combining material science innovations with procedural refinements to address long-standing clinical pain points. These systems aim to provide secure tissue approximation during the critical healing window while minimizing long-term foreign body presence; this dual objective is shaping surgeon preference, perioperative protocols, and procurement discussions. Over recent clinical cycles, designers and manufacturers have focused on achieving predictable degradation kinetics, reliable mechanical performance during early tissue ingrowth, and compatibility with evolving minimally invasive techniques.Clinicians are evaluating fixation choices not only on immediate procedural metrics such as deployment reliability and handling characteristics but also on downstream considerations including reduced chronic pain, ease of imaging, and implications for reoperation. In parallel, hospitals and ambulatory centers are weighing total procedural efficiency, device reproducibility across operator skill sets, and supply chain dependability. As a result, product development paths increasingly reflect an integrated set of priorities encompassing device ergonomics, polymer science, regulatory strategy, and commercial support models.
The synthesis of these forces underscores the importance of evidence generation that aligns biomechanical performance with real-world clinical outcomes. Consequently, intelligence that combines clinical insights, material performance data, and stakeholder perspectives is essential for companies seeking to differentiate offerings and for health systems aiming to adopt fixation solutions responsibly and sustainably.
A deep examination of technological, clinical, and procedural shifts reshaping absorbable fixation use in hernia surgery across delivery systems and surgical approaches
Technological and procedural shifts are converging to reshape the absorbable fixation landscape, with implications for device design, surgeon adoption, and procurement models. Innovations in polymer chemistry are enabling more precise control of degradation profiles and mechanical behavior, allowing manufacturers to tune fixation systems to match tissue healing timelines. Concurrently, advances in deployment mechanisms and ergonomic design are improving consistency of placement, lowering technical variability between automated and manual options, and reducing procedure times in experienced hands.Procedural trends toward minimally invasive access have amplified interest in fixation devices that offer reproducible performance in constrained operative spaces. Laparoscopic and robotic approaches demand compact, reliable delivery systems that integrate seamlessly with platform-specific instrumentation, while open procedures continue to rely on straightforward, rapid fixation techniques. These diverging procedural preferences are prompting modular product strategies that balance universal design with dedicated solutions for specialized workflows.
Clinical priorities are also shifting toward patient-centric outcomes beyond recurrence prevention. Attention to chronic postoperative pain, mesh integration, and postoperative imaging compatibility is influencing both clinician choice and payer evaluations. As a result, the interplay between clinical evidence, surgeon experience, and hospital procurement policies is becoming more dynamic, incentivizing manufacturers to invest in robust clinical programs and real-world evidence generation to demonstrate differentiated value propositions.
A thorough analysis of the cumulative impact of United States tariffs implemented in 2025 on supply chains, procurement strategies, and clinical adoption of absorbable fixation systems
The tariff environment introduced in the United States in 2025 has added a new layer of complexity to procurement and supply chain planning for medical devices, including absorbable fixation systems. Tariff-driven cost pressures are prompting manufacturers and health systems to reevaluate sourcing geographies, component procurement strategies, and inventory policies. This recalibration often begins with a review of supplier footprints and culminates in tactical adjustments such as nearshoring, dual-sourcing, or renegotiated commercial terms with strategic suppliers.Procurement teams are increasingly incorporating tariff sensitivity into total cost-of-ownership assessments, recognizing that device list price is only one component of overall value. Inventory strategies have shifted toward more nuanced safety stock policies that balance service-level expectations against the financial implications of tariff exposure. Parallel to these operational responses, manufacturers are exploring product and packaging redesigns that reduce tariff classification risk or shift value-added activities to tariff-favorable jurisdictions.
Clinically, the tariff-induced adjustments can influence device selection by providers, particularly where alternatives with comparable clinical profiles exist from different supply chains. Consequently, commercial teams need to communicate the clinical differentiators that justify procurement choices and demonstrate how supply continuity will be protected. Ultimately, the tariffs have accelerated a broader industry emphasis on supply chain resilience, transparent supplier relationships, and scenario planning to maintain access to preferred absorbable fixation options under variable trade conditions.
Strategic segmentation insights synthesizing fixation types, surgical approaches, hernia types, material formulations, distribution channels, and end user dynamics for targeted planning
A granular view of product and customer segments clarifies where clinical needs and commercial opportunity intersect, and where companies should prioritize development and outreach activities. Based on fixation type, the landscape encompasses absorbable clip, absorbable glue, absorbable screw, and absorbable tacker, with the absorbable tacker category further differentiated into automated tacker and manual tacker options that present distinct ergonomic and training considerations for operating teams. Based on surgery approach, fixation solutions must be evaluated across laparoscopic, open, and robotic techniques, each presenting unique access dynamics, visualization patterns, and instrumentation compatibilities that affect device suitability and deployment reliability.Based on hernia type, clinical demand and device performance expectations vary across femoral, incisional, inguinal, umbilical, and ventral presentations, with tissue characteristics and recurrence risk profiles informing fixation strategy. Based on material type, familiar polymer families such as copolymer, polydioxanone, polyglycolic acid, and polylactic acid each bring trade-offs in degradation kinetics, tensile strength, and inflammatory response that influence both design decisions and clinician preference. Based on distribution channel, the pathways to market include direct sales channels, distributor partnerships, and online platforms, each requiring tailored commercial approaches in terms of training, inventory support, and contracting flexibility. Based on end user, adoption dynamics differ across ambulatory surgery centers, clinics, and hospitals, where reimbursement structures, procedural volume, and purchasing governance shape purchasing behavior and demand predictability.
Synthesizing these intersecting segmentation axes reveals areas where product differentiation, clinician education, and channel strategy can be coordinated to address unmet needs, reduce variability in outcomes, and create defensible commercial positioning. Recognizing how fixation type aligns with surgical approach and hernia presentation, and how material selection influences both clinical outcomes and handling characteristics, will be central to prioritizing development roadmaps and sales strategies.
A regional perspective that decodes demand drivers, regulatory environments, and care delivery nuances across the Americas, Europe Middle East Africa, and Asia Pacific
Regional dynamics materially influence clinical practice patterns, regulatory expectations, and commercial pathways for absorbable fixation systems. In the Americas, health systems and providers are focused on aligning clinical outcomes with cost-efficiency, and procurement decision-making tends to emphasize evidence that links device features to patient-centric endpoints and operating-room efficiency. In Europe, Middle East & Africa, regulatory frameworks and public procurement mechanisms introduce heterogeneity that requires flexible market access strategies and localized clinical engagement. In Asia-Pacific, rapid adoption of minimally invasive platforms in several markets is creating demand for devices that are compatible with diverse procedural ecosystems and competitive procurement environments.Across regions, reimbursement policies, hospital consolidation patterns, and the maturity of ambulatory surgery pathways shape where and how fixation systems are introduced and scaled. Regional clinical societies and key opinion leaders play a pivotal role in shaping practice patterns and accelerating or tempering adoption, depending on the strength of local evidence and training programs. Supply chain resilience considerations, including regulatory approvals and customs processes, also exert region-specific effects on product availability and procurement timelines.
Consequently, a nuanced regional strategy that integrates regulatory planning, evidence generation, and channel partnerships is required to translate product innovation into sustainable adoption. Tailoring clinical messaging and commercial support to regional care delivery models will be essential to unlocking opportunities and managing the operational realities of cross-border supply and distribution.
Competitive intelligence synthesizing product portfolios, R&D focus, partnership strategies, and commercialization approaches among leading medical device firms in absorbable fixation
Competitive positioning in absorbable fixation systems is driven by the intersection of product performance, evidence generation, and commercialization excellence. Market participants are differentiating through focused investments in polymer science to refine degradation behavior and implant-host interactions, as well as through engineering innovations that improve deployment ergonomics and consistency across operator skill levels. Firms with integrated clinical programs that combine randomized evidence, registries, and real-world outcomes data are better positioned to influence clinician preference and procurement decisions.Strategic partnerships are also shaping competitive dynamics, including collaborations with platform manufacturers, surgical training organizations, and distribution partners that expand access and support adoption. Companies that offer comprehensive training, strong post-market surveillance, and responsive field support tend to secure deeper institutional relationships and more rapid integration into care pathways. Additionally, those that invest in modular product families-capable of addressing multiple surgical approaches and a range of hernia types-can better respond to varied clinician workflows and purchasing models.
Operational excellence in manufacturing and supply chain management remains a key differentiator, particularly in the wake of trade policy changes and heightened attention to continuity of supply. Organizations that demonstrate transparent supplier relationships, robust quality systems, and the capacity to adapt component sourcing to shifting regulatory and tariff landscapes will be seen as lower-risk partners by large purchasers and health systems.
Targeted strategic recommendations for industry leaders to optimize product development, procurement resilience, clinical engagement, and commercialization in absorbable fixation
Industry leaders should adopt a multidimensional strategy to capitalize on clinical demand while mitigating commercial and operational risks. First, prioritize targeted clinical evidence generation that links polymer selection and fixation mechanics to patient-centered endpoints, such as pain reduction and functional recovery, enabling procurement teams to evaluate value beyond unit price. Second, design product portfolios with modularity in mind, ensuring that fixation systems are interoperable across laparoscopic, open, and robotic workflows to broaden applicability and ease adoption across diverse surgical settings.Third, fortify supply chain resilience by diversifying component sourcing, exploring nearshoring opportunities where feasible, and establishing contingency inventory agreements with key partners; transparency in supplier relationships will also reduce procurement friction. Fourth, invest in clinician-facing education and proctoring programs to reduce variability in deployment technique, thereby improving real-world outcomes and facilitating faster institutional uptake. Fifth, tailor commercial approaches to regional realities by aligning regulatory filings with local evidence priorities and by deploying channel strategies that reflect the purchasing behaviors of ambulatory surgery centers, clinics, and hospitals.
Finally, strengthen post-market surveillance and feedback loops to accelerate iterative improvement in product design and to provide purchasers with assurance around long-term safety and performance. By integrating clinical, operational, and commercial levers, organizations can build durable competitive advantage and support responsible diffusion of absorbable fixation technologies into clinical practice.
A transparent research methodology outlining primary and secondary data collection, expert validation, and analytical frameworks underpinning insights into absorbable fixation systems
The analysis underpinning these insights combined a structured review of peer-reviewed clinical literature, regulatory filings, and device labeling with primary qualitative interviews of operating surgeons, procurement leaders, and device engineers. Evidence synthesis prioritized peer-reviewed randomized trials, prospective registries, and biomechanical testing reports to align mechanical performance attributes with clinical outcomes and usability findings. Regulatory and tariff implications were evaluated through a review of public policy notices, customs classifications, and observed changes in procurement behavior reported by health system supply chain professionals.Primary research included interviews conducted with stakeholders across a range of provider types to capture differential needs among ambulatory surgery centers, clinics, and hospitals, and to understand how surgical approach and hernia presentation influence fixation choice. The methodology triangulated interview insights with device technical specifications and product deployment data to ensure that commercial and clinical recommendations reflect both articulated needs and observed device performance. Quality assurance was maintained through expert validation sessions and iterative review cycles with clinical advisors to confirm the interpretive coherence of the findings.
This mixed-methods approach ensured that recommendations are grounded in both empirical evidence and practical considerations relevant to clinicians, procurement teams, and commercial leaders. The methodology also emphasized transparency in data sources and limitations, facilitating informed decision-making while acknowledging areas that warrant further investigation or localized validation.
A concise conclusion synthesizing strategic priorities, resilience imperatives, and clinical adoption pathways for absorbable fixation systems in modern hernia care
The collective evidence points to a field in active transition, shaped by materials innovation, procedural evolution, and heightened attention to supply chain and procurement dynamics. Absorbable fixation systems are positioned to play a meaningful role in modern hernia repair pathways when device selection is aligned with surgical approach, hernia presentation, and institutional priorities. Success for manufacturers and providers alike will depend on integrating robust clinical evidence with pragmatic considerations of usability, cost of ownership, and supply continuity.Looking ahead, organizations that align product design with clinician workflows, invest in outcome-focused evidence, and prioritize resilient supply and distribution models will be best placed to deliver sustainable value. Cross-disciplinary collaboration among engineers, clinicians, and procurement experts will accelerate the translation of material and deployment innovations into measurable improvements in patient care. In the near term, careful attention to regional regulatory nuances and tariff-driven procurement realities will be required to maintain product availability and support adoption across diverse healthcare settings.
In sum, the pathway to broader, responsible uptake of absorbable fixation systems lies in evidence-led innovation, operational resilience, and targeted commercialization that respects the clinical and institutional contexts in which these devices are deployed.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Absorbable Fixation System for Hernia Repair Market
Companies Mentioned
- B. Braun SE
- Becton, Dickinson, and Company
- BioHealth Medical Tech
- CONMED Corporation
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson Services, Inc.
- LiNA Medical A/S
- Lotus Surgicals Pvt Ltd
- Medtronic plc
- Meril Life Science
- pfm medical gmbh
- Suture Planet
- Teleflex Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
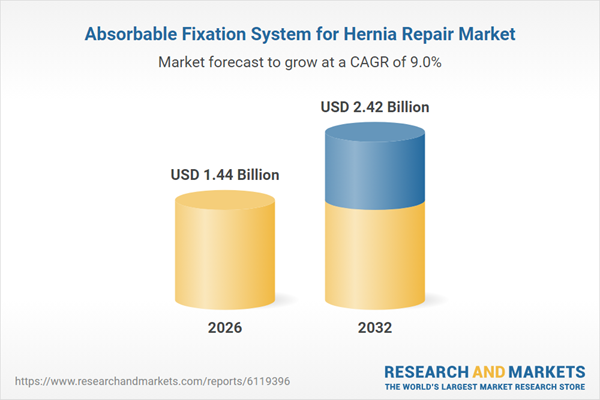
| Estimated Market Value ( USD | $ 1.44 Billion |
| Forecasted Market Value ( USD | $ 2.42 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |