Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive introduction to the evolving role of prefilled polymer syringes in modern therapeutic delivery and clinical workflow optimization
Prefilled polymer syringes have emerged as a pivotal component in modern injectable delivery strategies, combining material innovation with design refinements to meet clinical, logistical, and patient-centric demands. This introduction frames the technology’s role in streamlining administration workflows, improving dosing accuracy, and enhancing cold-chain resilience by leveraging polymer options that balance clarity, strength, and chemical compatibility. Over recent years, stakeholders across pharmaceutical development, hospital procurement, and home care delivery have increasingly prioritized devices that reduce waste, minimize contamination risks, and support self-administration paradigms.The near-term environment for these devices emphasizes integration with biologics and chronic care management systems, with manufacturers iterating on features such as safety mechanisms, luer interfaces, and capacity gradations to align with therapeutic profiles. Concurrently, regulatory authorities are refining device compatibility and extractables/leachables guidance, prompting manufacturers to invest in material characterization and robust quality management systems. As a result, product roadmaps are increasingly evidence-driven, with cross-functional teams coordinating clinical usability testing, material science validation, and scalable manufacturing planning to ensure market readiness and clinician acceptance.
How material advances, device safety innovations, and shifting care delivery models are jointly reshaping prefilled polymer syringe product and supply chain strategies
The landscape for prefilled polymer syringes is transforming under several converging forces that reshape product design, supply chain architecture, and commercial trajectories. Advances in polymer science have enabled the development of cyclic olefin copolymers, polycarbonates, and polypropylene formulations that deliver improved barrier properties, dimensional stability, and compatibility with a broader range of biologics. At the same time, device-level innovation such as enhanced safety syringes, auto-disable mechanisms, and varied luer configurations is responding to heightened priorities around needle-stick prevention and precise dose delivery.Simultaneously, shifts in healthcare delivery models are accelerating demand for home-based administration and ambulatory care solutions, prompting manufacturers to emphasize ergonomics, simplified activation, and patient-friendly labeling. Regulatory intensification around material safety and sterilization validation encourages early engagement between material scientists and clinical teams to de-risk product acceptance. Finally, digitalization and smart packaging trends are fostering pilot integrations that pair prefilled syringes with traceability systems and temperature-monitoring solutions, reinforcing the device’s role within broader cold-chain and pharmacovigilance frameworks.
Assessing the cumulative operational and sourcing consequences of United States tariff adjustments in 2025 and the resulting strategic supply chain responses
In 2025, tariff policy developments in the United States introduced new cost considerations and sourcing complexities that reverberate across the prefilled polymer syringe ecosystem. Increased levies on specific polymer imports and certain medical device categories created immediate incentives for supply chain reprioritization, encouraging manufacturers and distributors to re-evaluate supplier relationships and to accelerate nearshoring or domestic-sourcing strategies. As a result, procurement teams redirected qualification efforts toward regional suppliers with proven regulatory compliance and established sterilization capabilities to mitigate lead-time uncertainty and customs-related disruptions.Beyond immediate procurement adjustments, the policy environment prompted device developers to reassess cost structures and explore design modifications that reduce dependence on tariff-affected components. Contract manufacturers began expanding dual-sourcing approaches and investing in flexible production lines to absorb variability in input costs. In parallel, payers and large institutional buyers intensified negotiations on total cost of ownership metrics, factoring in logistics resilience and supplier stability. These combined actions have encouraged a more diversified and risk-aware supply base, helping stakeholders sustain continuity of care while navigating elevated trade-related complexities.
Deep segmentation insights showing how application, material, syringe form, capacity, end user, and distribution channel intersect to drive tailored product strategies
A nuanced understanding of segmentation dynamics reveals how clinical use cases, material selection, syringe form factor, capacity range, end-user setting, and distribution pathways interact to shape adoption and product positioning. Based on application, clinical priorities vary widely across anesthesia, diagnostic procedures, insulin delivery, and vaccine injection, with insulin delivery requiring differentiation between Type One delivery and Type Two delivery modalities and vaccine injection split between COVID-19 vaccine and influenza vaccine use cases; devices intended for anesthesia focus on precision and compatibility with anesthetic agents, while diagnostic procedures emphasize sample integrity and low dead-space configurations. Based on material type, performance trade-offs emerge between cyclic olefin copolymer options prized for low extractables and clarity, polycarbonate variants offering high impact resistance, and polypropylene choices that provide cost efficiency and chemical resistance; these material decisions influence sterilization approaches and labeling claims.Based on syringe type, regulatory and user-safety drivers shape demand for auto disable designs that prevent reuse, luer lock systems that provide secure connections, luer slip variants facilitating rapid assembly, and safety syringes engineered to reduce needlestick injury risk, with each format requiring specific user training and device validation. Based on capacity, clinical workflows dictate preference among 1 mL devices for precise microdosing, 2.5 mL and 5 mL volumes for intermediate therapeutic regimens, and 10 mL syringes for larger-volume administrations or irrigation tasks. Based on end user, institutional demands differ across ambulatory care centers, clinics, home care settings, hospitals, and research laboratories, with hospitals and research environments emphasizing regulatory traceability while home care settings prioritize ease of use and portability. Based on distribution channel, procurement pathways span hospital pharmacies, medical distributors, online pharmacies, and retail pharmacies, each introducing distinct logistics, inventory management practices, and regulatory compliance checkpoints. Taken together, these intersecting segmentation lenses illustrate how product development, regulatory strategy, and commercialization plans must be tailored to specific combinations of application, material, type, capacity, end user, and distribution context.
Regional dynamics and strategic imperatives across Americas, Europe Middle East & Africa, and Asia-Pacific that influence manufacturing, regulation, and procurement choices
Regional dynamics continue to influence manufacturing footprints, regulatory pathways, and demand patterns for prefilled polymer syringes, shaping where companies invest in capacity and support services. In the Americas, procurement tends to favor suppliers with strong regulatory track records and comprehensive cold-chain logistics, and the region remains a focal point for advanced biologics and chronic disease therapies that prioritize patient-centric delivery systems. In Europe, Middle East & Africa, fragmented regulatory frameworks and heterogeneous health system procurement processes require flexible market entry strategies, often combining local regulatory experts with regionally aligned manufacturing partners to satisfy diverse clinical and commercial requirements.Asia-Pacific presents divergent but rapidly maturing markets where domestic manufacturing capabilities are expanding, regulatory harmonization initiatives are progressing, and demand for cost-effective, scalable solutions is rising, particularly in high-volume vaccine programs and diabetes management. Across all regions, cross-border partnerships, contract manufacturing expansions, and targeted regulatory engagement prove essential to navigating local qualification standards and to ensuring timely access to critical therapies delivered via prefilled polymer syringes. Consequently, firms must balance global design standardization with region-specific adaptations to meet clinician expectations and supply chain realities.
Competitive landscape and corporate strategies revealing how vertical integration, material partnerships, and contract manufacturing shape leadership in prefilled polymer syringe solutions
Key industry participants demonstrate a range of approaches to material science, device engineering, and supply chain orchestration, with leaders investing in end-to-end capabilities that span polymer development, device assembly, sterilization, and regulatory support. Several companies are pursuing vertical integration to secure critical inputs and to enhance control over quality and traceability, while others are concentrating on specialized niches such as safety syringes or high-clarity polymer systems to capture specific clinical segments. Partnerships between polymer suppliers and medical device assemblers have become more strategic, enabling co-development of materials tailored to biologic compatibility and stability requirements.Contract manufacturing organizations expanded flexible production lines and validation services to support fast-track clinical programs and large-scale immunization initiatives. Meanwhile, distributors and hospital procurement groups are enhancing vendor qualification frameworks and digital ordering systems to reduce lead times and improve inventory visibility. Across the competitive landscape, differentiation increasingly depends on proven material characterization data, robust human factors evidence, and demonstrated supply continuity. Companies that align engineering rigor with regulatory agility position themselves to serve complex therapeutic categories and emerging care delivery models more effectively.
Actionable strategic recommendations for manufacturers and supply chain leaders to enhance material qualification, diversify sourcing, and strengthen user-centered device design
Leaders in the prefilled polymer syringe space should adopt a proactive posture that aligns product innovation with supply chain resilience, regulatory foresight, and user-centered design principles. First, prioritize material qualification programs that produce demonstrable extractables/leachables and stability data early in development to streamline regulatory submissions and to accelerate clinical adoption. Second, diversify supplier networks and qualify alternate polymer and component sources to reduce vulnerability to regional trade disruptions and tariff fluctuations, while investing in dual-sourcing strategies for critical sterilization and packaging services.Additionally, integrate human factors testing and real-world usability studies to validate safety features and to optimize labeling for diverse end-user settings, including home administration. Expand collaboration with payers and large institutional buyers to articulate total cost of ownership narratives that encompass logistics, training, and waste management. Finally, pursue modular manufacturing investments that allow rapid scale-up for vaccine or biologic programs while maintaining high-quality standards, and explore digital traceability integrations that improve lot tracking and pharmacovigilance outcomes. These actions will enable organizations to compete on reliability, regulatory readiness, and end-user value.
A robust multi-source research methodology combining expert interviews, regulatory review, material science literature, and usability validation to ensure actionable findings
This research synthesizes primary qualitative interviews with device engineers, procurement leads, and clinical stakeholders alongside a structured review of public regulatory guidance, peer-reviewed material science literature, and technical whitepapers on polymer performance and sterilization methods. The approach emphasized triangulation, using manufacturer disclosures, regulatory filings, and validated clinical usability studies to corroborate product feature claims and to assess adoption drivers across care settings. Secondary sources provided context on policy shifts and supply chain developments, enabling identification of systemic risks and adaptive strategies employed by market participants.Data collection prioritized verifiable technical metrics such as material compatibility, sterilization validation pathways, and design features related to safety and dose precision, while human factors evidence informed assessments of real-world usability. Analytical methods combined thematic synthesis for qualitative inputs with comparative evaluation frameworks to highlight strategic differentiators. Throughout, care was taken to validate conclusions against multiple independent sources and subject matter expert review to ensure accuracy and practical relevance for decision-makers exploring device development, procurement, or market entry initiatives.
Conclusive synthesis highlighting how technical, regulatory, and supply chain preparedness will determine long-term success for prefilled polymer syringe stakeholders
The cumulative evidence indicates that prefilled polymer syringes occupy a strategic position at the intersection of material innovation, patient-centric device design, and resilient supply networks. Progress in polymer formulations and device safety mechanisms has expanded the range of clinical applications, from high-precision insulin delivery to large-scale vaccination efforts, while evolving procurement practices and regional regulatory landscapes necessitate adaptable manufacturing and distribution models. Importantly, organizations that invest in rigorous material characterization, diversified sourcing, and human factors validation are better positioned to meet clinician expectations and to sustain continuity of care under market and policy pressures.Looking forward, the ability to integrate digital traceability, to align product features with specific clinical workflows, and to anticipate regulatory requirements will distinguish successful players. By blending technical rigor with pragmatic supply chain planning, stakeholders can convert emerging risks into competitive advantages and support broader public health objectives through reliable, user-friendly injectable delivery systems.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Prefilled Polymer Syringe Market
Companies Mentioned
- AptarGroup, Inc.
- Baxter International Inc.
- Becton Dickinson and Company
- Catalent, Inc.
- Gerresheimer AG
- Nipro Corporation
- SCHOTT AG
- Stevanato S.p.A
- Terumo Corporation
- West Pharmaceutical Services, Inc.
- Ypsomed Holding AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
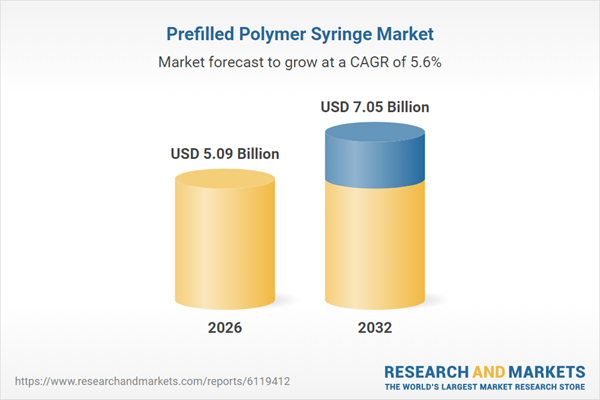
| Estimated Market Value ( USD | $ 5.09 Billion |
| Forecasted Market Value ( USD | $ 7.05 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |