Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to the general laboratory accessories ecosystem highlighting procurement priorities, operational dependencies, and compliance-driven considerations
The general laboratory accessories ecosystem underpins the daily operations of laboratories across academia, industry, clinical settings, and regulated manufacturing facilities. These items range from single-use consumables that support assay throughput to precision instruments that ensure data fidelity, and to personal protective equipment that safeguards personnel. Together they form a vital infrastructure layer that enables reproducible science, compliant production processes, and efficient workflows.In recent years, procurement behaviors and operational priorities have evolved: organizations emphasize traceability, supplier resilience, and sustainability in procurement decisions, while researchers and quality teams demand higher consistency and interoperability across instruments and consumables. Concurrently, regulatory scrutiny and biosafety imperatives have elevated the importance of validated supply chains and certified safety equipment. As a result, laboratory managers and procurement leads must balance cost-efficiency with continuity of supply and compliance requirements.
This report synthesizes those operational realities into a pragmatic narrative designed for decision-makers. It highlights the strategic trade-offs between single-source specialization and diversified vendor strategies, the operational benefits of standardized consumables and instruments, and the long-term implications of material selection for waste management and environmental compliance. By framing these themes in practical terms, the introduction sets the stage for deeper analysis of structural shifts, tariff impacts, segmentation nuances, regional behaviors, and strategic responses that follow.
How technological automation, regulatory traceability, and sustainability priorities are converging to reconfigure supplier relationships, procurement models, and product innovation
The landscape for general laboratory accessories is being reshaped by an alignment of technological, regulatory, and sustainability imperatives that together produce transformative outcomes for laboratories and suppliers. Advances in automation and laboratory information management systems are increasing demand for consumables and instruments that are compatible with high-throughput platforms, pushing manufacturers to prioritize standardization, modularity, and digital integration. As laboratories adopt more automated workflows, consumable design and instrument interfaces are converging to reduce error rates and accelerate sample processing.Regulatory evolution is a parallel force. Heightened expectations for traceability, validation, and documentation mean that suppliers must provide more detailed provenance data and support for quality systems. This regulatory pressure favors providers that can demonstrate consistent quality controls, certified materials, and audit-ready documentation. Consequently, buyers exhibit growing preference for vendors that offer end-to-end compliance support and validated product lines, rather than purely commoditized items.
Sustainability and circularity considerations are also redirecting product development and procurement choices. There is increasing interest in materials that reduce environmental impact without compromising performance, as well as in programs that enable responsible disposal or recycling of single-use items. When combined with cost containment pressures, these environmental drivers are prompting novel business models: consumable leasing, take-back schemes, and certified recycled-material options. Together, these technological, regulatory, and sustainability shifts are forcing a re-evaluation of long-standing supplier relationships and product selection criteria.
Operationally, these shifts manifest in greater collaboration between end users and suppliers during product development, more rigorous vendor qualification processes, and a premium on products that support interoperability and documentation. For laboratory managers, the practical implication is the need to integrate procurement strategies with quality and sustainability objectives, while for suppliers the implication is to invest in validation data, digital compatibility, and responsible materials sourcing to remain relevant in a rapidly professionalizing market.
Assessment of cumulative tariff-driven pressures on supply chains and procurement strategies highlighting supplier diversification, nearshoring, and contract protection measures
Trade policy developments and tariff measures implemented through 2025 have had cumulative effects on costs, supplier behavior, and sourcing strategies across the laboratory accessories space. Tariff adjustments exert pressure at multiple points in the value chain: they increase landed costs for imported instruments and consumables, create incentives for regional nearshoring of manufacturing, and catalyze contract renegotiations between distributors and end users. Over time, organizations have responded by diversifying supplier bases, re-evaluating product specifications to identify domestically sourced alternatives, and increasing inventory buffers to mitigate short-term volatility.Instruments with specialized components and precision tolerances are particularly sensitive to cross-border cost shifts, because manufacturing often relies on globalized sub-supplier networks. As a result, procurement teams have begun to separate commoditized items from strategic purchases, insulating critical instrument contracts through long-term agreements or local supplier partnerships while permitting more flexibility in lower-value consumable sourcing. This bifurcated approach allows organizations to protect essential capabilities while containing exposure to tariff-induced cost swings.
Tariffs also change the calculus for distributors and online channels. Distributors may absorb some cost increases to maintain competitive pricing, but sustained tariff pressure incentivizes consolidation of distribution networks and the development of private-label offerings sourced from tariff-favored jurisdictions. Online channels, especially those managed by manufacturers, have become more attractive as they give suppliers greater control over pricing and logistics, enabling dynamic adjustments to mitigate tariff impact.
Operational responses have included greater emphasis on supplier qualification for regional manufacturers, investment in dual-sourcing strategies, and scenario-based procurement planning that incorporates potential trade-policy shifts. Over time these practices improve supply chain resilience, although they can lead to higher working capital requirements and more complex vendor management. For laboratory leaders, the pragmatic takeaway is to assess supplier geographic exposure, validate alternative sources for critical items, and incorporate tariff scenarios into contractual and inventory planning to maintain continuity of operations.
Segment-level intelligence that connects product typologies, end-user requirements, distribution models, and material choices to procurement strategies and operational risk management
Insightful segmentation analysis reveals where performance constraints and growth opportunities intersect across product types, end users, distribution channels, and materials. Within product typologies, routine consumables such as microplates, petri dishes, pipette tips, test tubes, and vials remain foundational to laboratory throughput; their demand dynamics are shaped by preferences for specific formats such as 384 well and 96 well microplates, which align to differing assay types and automation platforms. Lab glassware categories including beakers, burettes, flasks, and test tubes continue to serve core wet-lab functions where heat resistance and chemical compatibility are critical, and choices between glass subtypes drive procurement decisions tied to thermal and chemical stability. Instruments such as balances, centrifuges, pH meters, and spectrophotometers represent strategic capital purchases that require lifecycle planning and service provisions, while lab plasticware options like bottles, petri dishes, tubes, and vials offer cost and disposability trade-offs that laboratories manage through inventory policies. Safety equipment spanning fume hoods, gloves, goggles, and lab coats is evaluated through a risk-management lens, prioritizing certified performance and ergonomics.Different end-user groups impose distinct requirements that influence product design and procurement practices. Academic and research settings, including government labs, private research institutes, and universities, prioritize flexibility, reproducibility, and budget-conscious options; biotechnology firms balance production, quality control, and R&D needs with an emphasis on scalable consumables and validated instrumentation; chemical and industrial laboratories select materials and instruments optimized for corrosive or high-temperature processes; clinical diagnostics units emphasize traceability, lot consistency, and regulatory-ready documentation; food and beverage laboratories require suitable materials for hygiene and contamination control; pharmaceutical organizations allocate procurement across production, quality control, and R&D with stringent validation and supplier qualification standards. These end-user distinctions drive demand for configurable product offerings, certification packages, and after-sales service structures.
Distribution channels also shape accessibility and buying behavior. Direct sales channels offer customization, integrated service agreements, and bulk contracting advantages for institutional purchasers, whereas distributors, including regional and wholesale partners, provide logistical reach and localized inventory management for diverse buyers. Online channels, encompassing company websites and e-commerce platforms, improve speed and transparency in purchasing low-value, high-volume consumables, while retail outlets serve immediate replacement needs. Each channel demands different support infrastructures, from technical field service for instruments sold direct to streamlined fulfillment systems for high-volume consumables sold online.
Material selection remains a critical axis of differentiation and regulatory compliance. Glass variants such as borosilicate and soda-lime are chosen for their respective thermal and chemical properties, while metals like aluminum and stainless steel are prioritized for durability and corrosion resistance in durable equipment. Paper-based products including filter papers and wipes address sample preparation and cleanliness, and plastics such as polypropylene, polystyrene, and PVC continue to dominate single-use items with trade-offs in chemical compatibility and environmental footprint. Rubber materials, notably latex and nitrile, are central to gloves and sealing components, with nitrile increasingly preferred for chemical resistance and allergy mitigation. Understanding how product type, end-user needs, distribution pathways, and material properties interrelate enables procurement teams to align specifications with operational objectives and risk tolerance.
Regional differentiation and strategic implications for procurement and supplier strategy driven by manufacturing footprints, regulatory regimes, and demand concentration
Regional dynamics create differentiated priorities and supply chain architectures across the Americas, Europe Middle East & Africa, and Asia-Pacific, each of which exhibits distinct policy environments, manufacturing footprints, and end-user mixes. In the Americas, emphasis on domestic manufacturing and nearshoring has strengthened in response to trade uncertainties, prompting buyers to prioritize suppliers with transparent origin labeling and local service networks. This region also features strong clinical and pharmaceutical demand clusters that exert premium requirements for validated consumables and rapid instrument serviceability.Across Europe Middle East & Africa, regulatory harmonization and rigorous compliance standards elevate the importance of certified materials and documented quality systems. Laboratories in this region often prefer suppliers who can demonstrate conformity with regional directives and provide robust technical support. Moreover, sustainability legislation and corporate environmental commitments are increasingly influencing procurement decisions, driving suppliers to offer recyclable materials and take-back programs.
The Asia-Pacific landscape is characterized by a robust manufacturing base combined with rapidly escalating research and industrial activity. Local production capabilities provide advantages in cost and lead times for many consumables and plasticware, while demand for high-precision instruments is concentrated in advanced research hubs and pharmaceutical centers. Cross-border logistics and export controls shape distributor strategies, and rapid urbanization supports expanding clinical diagnostics and industrial testing segments. Collectively, these regional differences imply that global suppliers must adopt differentiated go-to-market approaches, balancing centralized product platforms with locally adapted service, compliance, and sustainability offerings to meet varied regional requirements.
Competitive dynamics and supplier value propositions emphasizing validation, after-sales service, distribution partnerships, and business model innovation for sustained differentiation
Competitive positioning among suppliers reflects a balance of product breadth, technical support capabilities, and the ability to demonstrate documented quality. Leading players emphasize integrated propositions that combine validated consumables, calibrated instruments, and safety solutions with demonstrable after-sales service and warranty programs. In instrument categories, companies that offer field service networks, remote diagnostics, and calibration support maintain a premium position because these services reduce downtime and protect data integrity. For consumables, vendors that provide lot-level traceability, certifications, and compatibility data for automated platforms secure preference among high-throughput laboratories.Distributors and e-commerce platforms are consolidating their roles as logistical partners that extend manufacturers’ reach. Strategic distributors that offer localized inventory management, just-in-time delivery, and bundled service agreements create differentiated value, particularly for multi-site organizations. At the same time, niche suppliers that focus on specialized materials or safety equipment leverage technical differentiation and compliance credentials to serve regulated end users.
To sustain competitive advantage, companies are investing in R&D for improved materials, validation data packages, and digital tools that facilitate product selection and integration. Partnerships between instrument manufacturers and consumable suppliers are becoming more common to ensure interoperability and consistent performance across automated workflows. Additionally, business model innovation such as subscription services for consumables, managed inventory programs, and reuse or recycling schemes is gaining traction as companies pursue recurring revenue and closer customer engagement. These strategic moves shape procurement conversations and raise benchmarks for reliability, transparency, and total cost of ownership.
Practical and prioritized steps procurement and operations leaders can implement to strengthen supply resilience, compliance posture, and sustainability outcomes
Leaders in laboratory operations and supplier organizations should adopt pragmatic, actionable steps to enhance resilience, cost-effectiveness, and sustainability. First, procurement and engineering teams should implement supplier segmentation that distinguishes critical instrument and safety equipment providers from high-volume consumable suppliers; this enables tailored contracting and risk mitigation strategies. Second, develop and maintain validated alternate sources for key consumables and instrument components to reduce single-supplier exposure while preserving quality and regulatory compliance. These actions must be supported by contractual terms that clarify lead times, quality thresholds, and contingency provisions.Third, integrate tariff and trade-policy scenario planning into procurement cycles and budgeting exercises. Establish threshold triggers for switching to regional suppliers or increasing safety stock when trade-policy volatility rises. Fourth, prioritize suppliers that provide robust traceability and certification documentation, particularly for clinical, pharmaceutical, and regulated manufacturing contexts; such documentation reduces audit risk and accelerates qualification timelines. Fifth, pursue material- and lifecycle-focused procurement policies that evaluate total environmental impact, enabling selection of products that align with corporate sustainability goals and regulatory requirements.
Finally, invest in digital procurement tools that improve visibility across inventory, supplier performance metrics, and contract compliance. These systems enable data-driven decisions, streamline supplier qualification, and support predictive maintenance schedules for instruments. Collectively, these recommendations will help organizations fortify operations against supply disruption, optimize cost structures, and align procurement choices with broader organizational priorities.
Transparent research methodology combining stakeholder interviews, regulatory and supply-chain analysis, and scenario-based evaluation to derive actionable procurement and operational insights
The research approach combined qualitative stakeholder engagement with targeted secondary analysis to form a robust basis for strategic insights. Primary engagement included structured interviews with procurement managers, laboratory directors, quality assurance leads, and distributor executives across academic, industrial, and clinical settings to capture real-world procurement challenges, supplier performance expectations, and evolving operational priorities. These interviews were supplemented by technical consultations with laboratory engineers and compliance specialists to validate product performance attributes, validation needs, and service requirements.Secondary analysis comprised review of regulatory documentation, trade-policy announcements, and manufacturing supply-chain mappings to understand geographic exposure and compliance implications. Publicly available technical standards and certification requirements were used to evaluate safety equipment and material suitability, and manufacturer specifications informed comparisons of instrument features, calibration intervals, and after-sales service models. Data synthesis prioritized triangulation across sources to ensure consistency and to highlight areas of divergence between stakeholder perceptions and documented supplier capabilities.
Finally, scenario-analysis techniques were applied to evaluate the operational impacts of tariff changes and supply disruptions, emphasizing procurement and inventory responses rather than predictive economic modeling. The methodology focused on translating evidence into actionable strategies tailored for procurement, operations, and executive stakeholders while maintaining transparency about assumptions and evidence sources.
Concise conclusion emphasizing integration of procurement, compliance, sustainability, and supplier strategy to enhance continuity, data integrity, and operational excellence
In closing, the general laboratory accessories domain is experiencing a phase of professionalization where operational resilience, regulatory conformity, and sustainability considerations increasingly drive procurement choices. Technological adoption and automation intensify demands for standardized, validated consumables and instrument interoperability, while trade-policy dynamics press organizations to reassess sourcing geographies and contractual protections. These concurrent pressures favor suppliers that can demonstrate rigorous quality systems, responsive service networks, and transparent material provenance.For laboratory leaders, the imperative is clear: integrate procurement strategy with quality, compliance, and sustainability objectives, and prioritize supplier relationships that deliver documentation, service, and regional resilience. For suppliers, the pathway to relevance lies in investing in validation data, digital enablement, and business models that reduce buyer risk and enhance lifecycle value. When executed coherently, these measures will improve operational continuity, protect data integrity, and support long-term institutional goals for safety and environmental responsibility.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China General Lab Accessories Market
Companies Mentioned
- Abbott Laboratories
- Agilent Technologies, Inc.
- Avantor, Inc.
- Beckman Coulter
- Bio-Rad Laboratories, Inc.
- Bruker Corporation
- Cole-Parmer
- Corning Incorporated
- Danaher Corporation
- Eppendorf AG
- Hettich
- Illumina
- Merck KGaA
- Mettler-Toledo
- Mindray
- PerkinElmer, Inc.
- QuidelOrtho
- Roche
- Sartorius AG
- Shimadzu
- Siemens AG
- Thermo Fisher Scientific Inc.
- VWR International
- Waters Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
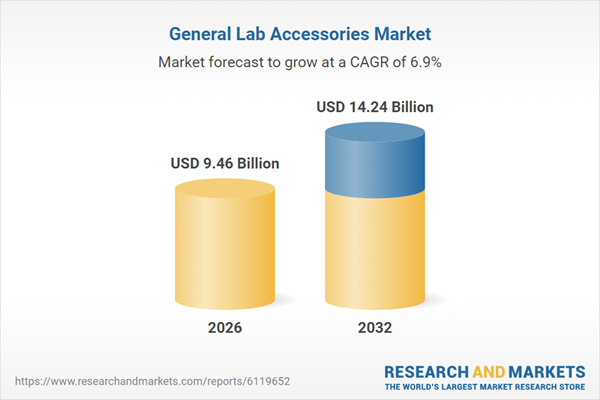
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 9.46 Billion |
| Forecasted Market Value ( USD | $ 14.24 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |