Speak directly to the analyst to clarify any post sales queries you may have.
Concise introduction to the clinical role, technological evolution, and ecosystem implications of transcranial direct current stimulation as an emerging neuromodulation modality
This executive summary introduces the landscape for transcranial direct current stimulation (tDCS), a noninvasive neuromodulation modality that has progressed from experimental laboratory protocols to pragmatic clinical and consumer applications. Initially developed to modulate cortical excitability through low-intensity electrical currents, tDCS now occupies a strategic position at the intersection of cognitive enhancement and therapeutic neurology. Technological refinement in electrode design, stimulation waveforms, and device ergonomics has expanded feasible use cases while simultaneously prompting new questions about standardization, safety, and efficacy across populations.As clinical trials accumulate and home-use devices proliferate, stakeholders including clinicians, researchers, device manufacturers, and payers are recalibrating their approaches to evidence generation, regulatory compliance, and commercialization. Moreover, adoption patterns are shaped by reimbursement frameworks, clinician training pipelines, and patient acceptability, which in turn influence product design and distribution strategies. This introduction sets the stage for deeper analysis by outlining the underlying mechanisms, typical clinical indications, and the evolving ecosystem of suppliers and service providers. By anchoring subsequent sections in this foundational view, readers will better appreciate how technological, regulatory, and market forces collectively determine adoption trajectories and commercialization opportunities.
How converging evidence standards, hybrid delivery models, and advanced personalization technologies are reshaping clinical adoption and commercialization pathways for tDCS devices
The past several years have seen transformative shifts that reframe how tDCS is researched, regulated, and commercialized. First, the maturation of high-quality randomized controlled trials and meta-analytic syntheses has strengthened the evidence base for specific therapeutic indications while simultaneously revealing heterogeneity in outcomes tied to dosing parameters, electrode montages, and patient selection. Consequently, stakeholders are moving away from one-size-fits-all approaches toward precision protocols that account for neuroanatomical and phenotypic variability.Second, there has been an operational shift in delivery models: devices optimized for clinic settings coexist with portable, wearable systems designed for supervised home use. This duality is driving innovation in remote monitoring, digital adherence platforms, and hybrid care pathways that integrate telehealth touchpoints with in-person clinical oversight. Third, regulatory pathways are evolving in parallel with product innovation; regulators are increasingly demanding more rigorous safety and efficacy data while also creating conditional or accelerated access tracks for devices with compelling unmet clinical need.
Finally, the convergence of neuromodulation with data-driven approaches, such as closed-loop systems and machine learning-based personalization, is changing expectations about clinical outcomes and user experience. Taken together, these shifts create new windows for competitive differentiation but also raise the bar for evidence generation, quality assurance, and post-market surveillance.
Assessment of how the 2025 United States tariff measures are catalyzing supply chain restructuring, pricing responses, and strategic regional manufacturing choices across the tDCS value chain
The imposition of new tariff structures within the United States in 2025 has introduced a set of cumulative effects that reverberate across supply chains, pricing strategies, and market entry decisions. Tariff-related cost increases on imported components and finished devices have compelled manufacturers to reassess sourcing strategies, spurring regional diversification of suppliers and renewed attention to domestic manufacturing capabilities. For many device producers, the immediate operational response has included renegotiation of supplier contracts, identification of alternate lower-cost component sources, and selective passing through of incremental costs to downstream purchasers.In addition to direct input-cost impacts, tariffs have altered competitive dynamics by affecting price parity between domestically produced units and imported alternatives. This change has implications for procurement decisions by hospitals and clinics that operate under constrained capital budgets. Furthermore, tariffs amplify the importance of value communication; manufacturers must more explicitly document clinical and economic benefits to justify price adjustments and to persuade procurement committees and payers to sustain investment in neuromodulation technologies.
Transitional strategies have emerged in response: some companies have accelerated investments in local assembly and certification, others have intensified focus on software and service bundles that differentiate offerings beyond hardware, and several manufacturers have reweighted their geographic go-to-market plans to emphasize regions with more favorable tariff or trade arrangements. As a result, tariffs are not merely a cost factor; they act as a catalyst for supply chain resilience, vertical integration, and strategic repositioning within the global tDCS ecosystem.
In-depth segmentation synthesis showing how application, end-user, product type, and distribution channel distinctions demand differentiated development, evidence, and commercialization strategies
A nuanced segmentation analysis reveals the heterogeneous drivers of demand and the specialization required to address distinct clinical and consumer needs. When analyzing the market through the lens of application, one observes that cognitive enhancement and neuropsychiatric disorders attract different evidence expectations and adoption pathways. Cognitive enhancement subdivides into attention and memory applications where user acceptability and ethical considerations shape product messaging. Neuropsychiatric disorders fragment into anxiety, depression, and schizophrenia, each presenting unique clinical endpoints, trial designs, and reimbursement dialogues. Pain management comprises chronic pain, migraine, and neuropathic pain, requiring tailored stimulation protocols and multidisciplinary care integration. Stroke rehabilitation separates into language recovery and motor recovery domains, where timing of intervention and rehabilitation workflows critically determine outcomes.Viewing end users clarifies distribution and support requirements: clinics, home care settings, hospitals, and research institutes each impose distinct service-level expectations. Home care further differentiates between organized home health agency deployments and individual users who seek consumer-friendly interfaces and clear safety guardrails. Research institutes split across academic institutions that prioritize mechanistic studies and pharmaceutical companies that focus on adjunctive therapy development. Product type segmentation shows similar divergence: portable devices, including handheld systems and wearable tDCS, prioritize usability and remote monitoring, while stationary clinical tDCS units emphasize protocol flexibility and integration with clinical workflows. Distribution channels likewise bifurcate into offline channels with distributors, hospital pharmacy networks, and specialty clinics that require channel-specific contracts, and online channels such as e-commerce platforms and manufacturer-direct sales that demand digital marketing, serviceable logistics, and clear warranty structures.
Taken together, these interlocking segmentations demonstrate that successful offerings will need to reconcile clinical rigor with user-centered design, channel-specific marketing, and post-sale support models that reflect the operational realities of each end-user cohort.
Comparative regional insights revealing how regulatory, reimbursement, cultural, and supply chain variations in the Americas, Europe Middle East & Africa, and Asia-Pacific shape adoption and commercialization
Regional dynamics exert a powerful influence on regulatory expectations, reimbursement pathways, clinical adoption rates, and supply chain logistics. In the Americas, robust clinician engagement, a strong tradition of clinical trials, and payer-driven reimbursement conversations create a market environment that favors well-documented clinical benefit and scalable distribution models. Meanwhile, Europe, Middle East & Africa presents a mosaic of regulatory frameworks and reimbursement approaches where country-level policies and health technology assessment processes determine the pace of adoption; here, targeted pilot programs and regional partnerships are often necessary to unlock institutional buy-in. In the Asia-Pacific region, rapid urbanization, increasing healthcare expenditure, and a growing emphasis on digital health solutions create fertile ground for both clinic-based and home-use tDCS offerings, although local regulatory pathways and distribution partner selection remain critical determinants of success.Across all regions, clinical acceptance is mediated by cultural attitudes toward neuromodulation, the available infrastructure for delivering rehabilitative and psychiatric care, and the degree to which digital health integration is supported by healthcare systems. Supply chain considerations also vary regionally: proximity to component suppliers, tariff exposure, and logistics capabilities influence lead times and cost structures. Finally, region-specific pilot outcomes and real-world evidence can serve as templates that accelerate wider adoption when adapted appropriately to local clinical and reimbursement contexts.
Critical company strategic archetypes and competitive behaviors that determine how medical device firms and startups capture clinical, home-use, and hybrid transcranial stimulation markets
Key company-level dynamics reflect divergent strategic approaches to product development, clinical evidence generation, and go-to-market execution. Established medical device firms tend to leverage deep regulatory experience and distribution networks to position clinical-grade, stationary tDCS systems within hospitals and specialty clinics, emphasizing protocol flexibility and integration with existing therapeutic workflows. At the same time, a cohort of innovative startups focuses on portable and wearable formats, seeking to capture home-use segments through intuitive design, remote monitoring capabilities, and subscription-based service models. Several organizations are differentiating through vertical integration of hardware, software, and clinical services to create bundled solutions that reduce friction for purchasers and enhance long-term customer engagement.Partnerships between device manufacturers and academic or clinical research centers are increasingly common, accelerating validation efforts and facilitating real-world evidence generation. Meanwhile, certain market participants prioritize reimbursement strategy early in product development, aligning clinical endpoints with payer requirements to streamline procurement discussions. Competitive positioning also involves intellectual property strategies around electrode technology, stimulation algorithms, and safety features. Finally, market entrants must balance speed to market with the need for robust post-market surveillance systems that capture safety and efficacy across diverse user populations, thereby sustaining regulatory compliance and clinician confidence.
Actionable recommendations for device developers, clinical leaders, and commercial teams to align evidence, product architecture, and distribution resilience for sustainable growth
Leaders in the tDCS domain should adopt a set of actionable priorities to sustain growth and manage evolving market risks. First, invest proactively in targeted clinical programs that align with high-priority applications and demonstrate reproducible outcomes; this approach enables clearer value propositions for clinicians and payers. Second, develop modular product architectures that accommodate both stationary clinical systems and portable wearable devices, thereby maximizing addressable use cases while controlling manufacturing complexity. Third, strengthen supply chain resilience by qualifying secondary suppliers, exploring regional assembly options, and modeling tariff exposures to protect margins and delivery timelines.In parallel, it is advisable to build robust digital ecosystems around hardware offerings, including remote monitoring, adherence tracking, and clinician dashboards that facilitate hybrid care models. Stakeholders should also prioritize regulatory engagement early, seeking constructive dialogues with authorities to clarify evidence requirements and to explore conditional access pathways. Commercially, tailoring distribution strategies by end-user segment-balancing direct sales, distributor partnerships, and digital channels-will improve market penetration and serviceability. Finally, establish rigorous post-market evidence programs that capture long-term outcomes and safety signals, thereby reinforcing trust among clinicians, patients, and payers and supporting favorable procurement decisions.
Rigorous mixed-method research approach combining primary interviews, literature synthesis, supply chain analysis, and expert validation to ensure robust and actionable findings
This analysis draws on a blended research methodology designed to triangulate qualitative insights and quantitative signals through multiple complementary approaches. Primary research consisted of structured interviews with clinicians, rehabilitation specialists, health system procurement leaders, and product executives to capture practical considerations around clinical protocols, device selection criteria, and service requirements. Secondary research synthesized peer-reviewed clinical literature, regulatory guidance documents, and white papers to map the current evidence landscape and to identify gaps in outcome consistency and safety reporting.Supply chain and tariff impact assessments were developed through supplier mapping, trade data review, and scenario analysis that explored alternative sourcing strategies and regional manufacturing implications. Competitive and company-level insights were derived from product literature, patent filings, and public disclosures, with emphasis on differentiating feature sets, integration capabilities, and commercialization models. Finally, findings were validated through expert panels and iterative review cycles to ensure that conclusions reflect current practice patterns, plausible regulatory trajectories, and realistic commercial constraints.
Concluding synthesis that emphasizes evidence-driven placement, supply resilience, and service-enabled differentiation as the pillars for scaling tDCS adoption across clinical and home environments
In conclusion, transcranial direct current stimulation occupies a dynamic and rapidly evolving intersection of neuroscience, medical device innovation, and digital health. The trajectory from laboratory research to routine clinical and home use is influenced by convergent forces: increasing evidence quality, hybrid delivery models, regulatory tightening, and supply chain realignment in response to tariff pressures. Segment-specific complexities-spanning cognitive enhancement, neuropsychiatric care, pain management, and stroke rehabilitation-require differentiated evidence strategies and user-centric product design. Likewise, end-user diversity and regional variations demand flexible commercialization and distribution approaches that can be adapted to local regulatory and reimbursement realities.Organizations that successfully integrate rigorous clinical validation, resilient manufacturing and sourcing, and compelling digital services will be best positioned to capture the expanding range of clinical and consumer opportunities. As stakeholders plan next steps, emphasis on strategic partnerships, post-market evidence collection, and clear articulation of value to clinicians and payers will be essential to accelerate adoption while maintaining safety and efficacy standards.
Table of Contents
17. ResearchStatistics
18. ResearchContacts
19. ResearchArticles
20. Appendix
Companies Mentioned
- Brainbox Ltd.
- Caputron LLC
- ElectroCore LLC
- Magstim Company Limited
- MagVenture A/S
- Mettler Electronics Corp.
- Mind Alive Inc.
- Neopraxis Pty Ltd
- NeuroCare Group GmbH (Nexstim Oyj)
- neuroConn GmbH
- Neuroelectrics Barcelona SL
- Neuroelectrics USA Inc.
- Neuronetics Inc.
- NeuroSigma Inc.
- Rogue Resolutions Ltd.
- Soterix Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
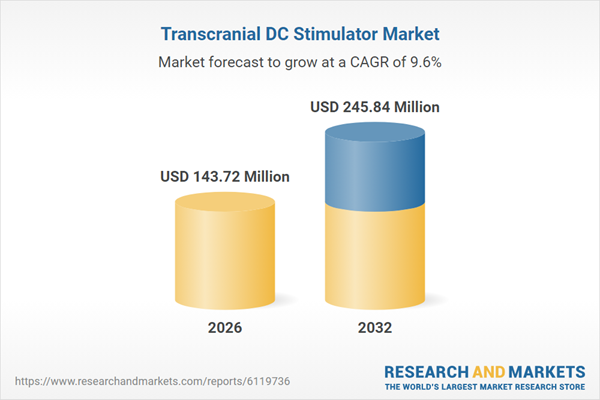
| Estimated Market Value ( USD | $ 143.72 Million |
| Forecasted Market Value ( USD | $ 245.84 Million |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |