Speak directly to the analyst to clarify any post sales queries you may have.
Why ≥ 5MHU CT tubes have become a strategic lever for uptime, image consistency, and lifecycle economics in modern CT operations
The ≥ 5MHU CT tube category sits at the intersection of clinical performance, operational resilience, and increasingly disciplined cost governance. As CT systems take on more demanding roles-ranging from trauma and stroke pathways to cardiac imaging and oncology staging-high-heat-capacity tubes are being asked to deliver faster throughput, consistent image quality, and dependable uptime under sustained duty cycles. That combination elevates tubes from a replaceable component to a strategic asset that influences scanner availability, patient flow, and service economics.At the same time, tube-related decisions are becoming more consequential because the broader ecosystem is changing. Hospitals are consolidating imaging networks, standardizing platforms, and enforcing tighter service-level expectations. OEMs are balancing innovation roadmaps with supply continuity and quality management, while independent service organizations expand capabilities to meet cost pressures. In this environment, ≥ 5MHU CT tubes represent a focal point where engineering constraints, clinical priorities, and procurement realities converge.
This executive summary frames what matters most right now: how the landscape is shifting, what the 2025 tariff environment implies for sourcing and pricing mechanics, where segmentation reveals actionable patterns, and how regional dynamics and competitive strategies are evolving. The goal is not to quantify the market, but to clarify decision paths and highlight the operational moves that separate reactive maintenance from strategic lifecycle management.
How protocol intensity, uptime expectations, predictive service, and supply resilience are redefining the ≥ 5MHU CT tube competitive landscape
The landscape for ≥ 5MHU CT tubes is being reshaped by a set of reinforcing shifts that are both technical and commercial. First, clinical protocols are intensifying thermal loads. Faster gantry rotations, wider detector coverage, and higher duty cycles in emergency and cardiac workflows increase the need for stable heat storage and dissipation. This is pushing stakeholders to scrutinize not only nominal MHU ratings but also real-world thermal management, anode cooling behavior, and consistency across repeated high-output scans.Second, reliability expectations are rising as CT becomes more central to care pathways that cannot tolerate downtime. Imaging departments increasingly manage scanner uptime like a production KPI, and tube performance is being evaluated through the lens of variability reduction. That shift favors suppliers and service models that can demonstrate predictable tube life behavior, robust failure analytics, and controlled installation practices rather than relying on average-life claims.
Third, the service model is evolving toward data-driven maintenance and lifecycle planning. Remote monitoring, tube usage analytics, and predictive triggers are increasingly used to time replacements, reduce unplanned failures, and align spares strategies with utilization profiles. As a result, the value proposition is moving from “a tube” to “tube performance over time,” including field support, logistics responsiveness, and standardized quality documentation.
Fourth, supply-chain resilience has become a board-level concern. Concentrated manufacturing capabilities, long lead times for specialized subcomponents, and stringent quality requirements create a risk profile that procurement teams can no longer treat as routine. Dual sourcing, qualification of alternates, and regional stocking strategies are being revisited, while OEMs weigh vertical integration and tighter supplier governance.
Finally, sustainability and compliance considerations are influencing choices in subtle but real ways. Energy efficiency, waste handling for end-of-life components, and transparent traceability expectations are increasingly tied to institutional procurement frameworks. Together, these shifts are transforming ≥ 5MHU CT tubes from a replacement cycle discussion into a broader strategy covering performance assurance, risk management, and total lifecycle stewardship.
What the 2025 U.S. tariff environment changes for ≥ 5MHU CT tubes: landed cost pathways, lead-time risk, and contracting discipline
The 2025 U.S. tariff environment adds a layer of complexity to ≥ 5MHU CT tube sourcing that extends beyond straightforward price impacts. Because CT tubes sit within a multi-tier supply chain-often involving specialized materials, precision subassemblies, and regionally concentrated manufacturing-tariffs can affect landed cost through several pathways. These include direct duties on finished components, indirect impacts on upstream inputs, and administrative burdens tied to classification, documentation, and compliance verification.One immediate effect is a greater emphasis on contract structure and price adjustment mechanics. Buyers are increasingly negotiating clearer terms around duty exposure, including how tariff-related changes are handled across the contract life. This is encouraging more disciplined cost breakdown discussions, stronger audit rights over surcharge logic, and tighter alignment between procurement and legal teams to prevent ambiguous pass-through practices.
Tariffs also influence lead-time risk. When policy changes introduce uncertainty, suppliers may adjust inventory positions, reroute logistics, or modify production allocations, which can create short-term fulfillment volatility. For imaging providers and service organizations, that volatility translates into greater pressure to refine spare tube planning, define acceptable substitution pathways, and maintain installation readiness to avoid extended downtime.
A further implication is the renewed attractiveness of regionalized strategies. Some stakeholders are evaluating the feasibility of shifting certain stages of assembly, testing, or distribution to reduce tariff exposure and strengthen supply continuity. Even when manufacturing geography cannot realistically change quickly, regional warehousing, bonded inventory arrangements, and alternative shipping routes can become practical tools to manage total landed cost and availability.
Importantly, tariffs also shape competitive behavior. Suppliers with more flexible footprints, diversified sourcing, or stronger compliance capabilities may use the environment to differentiate on reliability of supply rather than price alone. In parallel, buyers are revisiting qualification standards for third-party or remanufactured options where clinically and regulatorily appropriate, while ensuring that performance, safety, and traceability remain non-negotiable.
Overall, the 2025 tariff context encourages a shift from transactional purchasing to structured risk management. Organizations that treat tariff exposure as an operational variable-embedded in planning, contracting, and inventory policy-are better positioned to protect scanner uptime and maintain cost discipline without compromising clinical performance.
How product configuration, application intensity, end-user purchasing behavior, channel choice, and service models shape ≥ 5MHU CT tube decisions
Segmentation patterns in ≥ 5MHU CT tubes reveal that buying behavior is rarely uniform; it is shaped by how the tube is used, how replacement decisions are authorized, and what level of performance assurance the organization requires. When viewed through the lens of product configuration and heat management design, stakeholders tend to differentiate offerings by practical endurance under high-output protocols, stability of focal spot behavior under load, and the operational ease of maintaining consistent image quality across long duty cycles. This makes performance validation and quality documentation central to procurement conversations, especially where service partners must stand behind uptime commitments.Differences become even clearer when considered by application context and care setting. High-throughput environments prioritize predictable performance under repeated intensive scanning, while sites with intermittent peaks often focus on minimizing unplanned failures that disrupt scheduled capacity. In specialized clinical scenarios such as cardiac and emergency imaging, the tolerance for variability is low, which tends to elevate requirements for proven thermal behavior, repeatability, and rapid service response. As a result, organizations often align tube selection with protocol mixes and the operational cost of a scanner hour lost, rather than with component price alone.
End-user and channel dynamics further shape the segmentation story. Integrated delivery networks and large hospital groups frequently favor standardization, vendor governance, and harmonized service workflows, which can reduce variability in tube performance outcomes across sites. Independent facilities may prioritize flexibility in sourcing and faster replacement cycles, particularly when service coverage is constrained. Meanwhile, sourcing through OEM pathways versus third-party service or distribution channels often reflects different risk appetites and different internal quality thresholds for traceability, warranties, and post-installation support.
Finally, segmentation by service model highlights a critical strategic choice: reactive replacement versus lifecycle planning. Organizations that adopt structured lifecycle management-supported by usage analytics, remote monitoring, and planned spares-tend to reduce operational surprises and improve budget predictability. Those operating in a more reactive mode may experience higher disruption costs even if unit pricing is lower. In practice, segmentation insights indicate that the most successful strategies match tube configuration, quality assurance rigor, and service response capabilities to the clinical workload and the institution’s tolerance for operational risk.
Why Americas, Europe-Middle East-Africa, and Asia-Pacific differ in procurement priorities, service readiness, and uptime expectations for ≥ 5MHU CT tubes
Regional dynamics for ≥ 5MHU CT tubes are shaped by the interplay between installed base maturity, service infrastructure, procurement norms, and regulatory expectations. In the Americas, buyers often emphasize uptime guarantees, standardized service outcomes, and transparent documentation, reflecting the operational cost of downtime and the prominence of centralized procurement. This environment tends to reward suppliers and service partners that can deliver consistent lead times, robust warranty handling, and field support capable of minimizing disruption during tube replacement.Across Europe, the Middle East, and Africa, diversity in health system funding models and procurement frameworks creates a wide range of purchasing behaviors. In more standardized public procurement environments, formal qualification and compliance documentation can carry as much weight as technical performance claims. At the same time, regional service coverage and logistics reach become differentiators, particularly where cross-border supply and local installation capability determine how quickly a scanner can return to service. Sustainability and traceability considerations are also increasingly visible in procurement scoring, pushing suppliers to provide clearer end-of-life and materials-handling pathways.
In Asia-Pacific, demand patterns are influenced by a combination of rapid imaging capacity expansion in some markets and modernization of installed bases in others. High patient volumes in major urban centers elevate the value of thermal robustness and predictable tube life under intensive use. Meanwhile, supply continuity and service capability can vary significantly by country, encouraging a mix of OEM-aligned sourcing and localized service strategies. Training depth for installation and calibration, as well as access to qualified engineers, can directly affect realized tube performance and perceived supplier reliability.
When these regions are viewed together, a clear takeaway emerges: success depends on aligning product and service strategy with local operational realities. Organizations that map regional lead-time risk, service coverage constraints, and procurement requirements into a coherent sourcing approach are better positioned to secure uptime and maintain quality consistency across multi-site networks.
How leading ≥ 5MHU CT tube providers compete through quality systems, supply continuity, field service excellence, and pragmatic innovation that improves uptime
Competition in ≥ 5MHU CT tubes is characterized by a mix of OEM-aligned suppliers, specialized component manufacturers, and service-oriented organizations that emphasize availability and lifecycle support. The strongest participants tend to differentiate through repeatable performance in demanding protocols, quality systems that withstand audits, and field enablement that ensures installation consistency. In practice, the “company advantage” is often less about a single specification and more about the ability to deliver stable outcomes across manufacturing, logistics, and service touchpoints.A key theme in company strategies is tighter integration between tube supply and service execution. Organizations with strong installation training, standardized calibration practices, and data feedback loops can reduce early-life issues and improve customer confidence. This capability matters because real-world tube performance depends on more than component design; it also depends on handling, setup, cooling system compatibility, and the discipline of preventive maintenance.
Another differentiator is supply reliability under uncertainty. Companies that have diversified sourcing for critical subcomponents, maintain regionally positioned inventory, and operate mature compliance processes are better equipped to manage disruptions without cascading downtime at customer sites. Additionally, firms that can provide transparent documentation-covering traceability, conformity, and quality controls-are increasingly favored by hospital systems with formal vendor governance.
Innovation remains important, but it is being evaluated through a pragmatic lens. Buyers are looking for advances that translate into measurable operational benefits, such as better thermal management under repeated high-output scans, improved consistency that supports protocol standardization, and service models that reduce unplanned failures. The companies that communicate these benefits in operational terms, and that back them with responsive support, are more likely to earn long-term preferred status in high-utilization environments.
What industry leaders should do now: align tube choice to protocol intensity, contract for tariff resilience, standardize service, and govern suppliers by outcomes
Industry leaders can strengthen their ≥ 5MHU CT tube position by treating tube strategy as a reliability program rather than a replacement purchase. Start by aligning tube selection with protocol intensity and scanner utilization profiles, ensuring that specifications are validated against real operational duty cycles. This alignment is most effective when engineering, clinical leaders, and service managers jointly define acceptable variability thresholds for image quality and downtime, creating a shared decision framework.Next, embed tariff and supply uncertainty into contracting and inventory policy. Contract language should explicitly define how duty changes are handled, what documentation is required to justify surcharges, and how lead-time commitments are enforced. In parallel, organizations can reduce risk by setting spares strategies tied to utilization and criticality, positioning inventory closer to high-throughput sites, and defining qualified substitution pathways that preserve safety and compliance.
Service execution is a major lever. Standardize installation procedures, calibration practices, and post-installation verification to reduce preventable early failures and image variability. Where feasible, implement tube usage analytics and remote monitoring triggers to shift replacements from reactive to planned events. This not only protects uptime but also improves budgeting discipline and reduces the operational disruption associated with emergency swaps.
Finally, strengthen supplier governance with outcome-based scorecards. Track metrics such as lead-time adherence, documentation completeness, failure modes, and response speed, and use those insights in quarterly business reviews. Over time, this approach encourages suppliers to invest in the capabilities that matter most-consistent field outcomes and resilient supply-while giving buyers a defensible basis for standardization decisions across multi-site imaging networks.
How the research was built: triangulated primary interviews, technical and policy review, and segmentation-led synthesis for ≥ 5MHU CT tubes
This research was developed using a structured, triangulated methodology designed to reflect the realities of ≥ 5MHU CT tube decision-making without relying on simplistic assumptions. The process began with an extensive review of the technology and value chain, mapping how design constraints, manufacturing requirements, distribution models, and service execution influence performance outcomes. This framing ensured that subsequent analysis focused on decision drivers that materially affect uptime, quality consistency, and lifecycle management.Primary insights were gathered through interviews and consultations with stakeholders across the ecosystem, including roles spanning imaging operations, clinical engineering, procurement, service delivery, and supplier management. These conversations were used to validate practical pain points such as lead-time volatility, installation variability, documentation burdens, and the operational consequences of unplanned tube failures. Qualitative inputs were treated as directional signals and were cross-checked against other evidence to minimize single-source bias.
Secondary research included analysis of publicly available technical documentation, regulatory and compliance expectations, trade policy developments relevant to the 2025 tariff environment, and company-level materials such as product literature and quality statements. This information was used to contextualize competitive strategies, regional considerations, and the evolving expectations around traceability and lifecycle stewardship.
Finally, findings were synthesized through a segmentation and regional lens to highlight patterns in requirements and buying behaviors. Throughout, the approach emphasized internal consistency, practicality for decision-makers, and clear linkage between observed industry shifts and recommended actions. The result is a decision-oriented narrative that supports procurement, product planning, and service strategy discussions with defensible logic and operational relevance.
Where the market is headed: operational reliability, tariff-aware sourcing, and context-specific strategies will define success for ≥ 5MHU CT tubes
The ≥ 5MHU CT tube market environment is being shaped by intensifying clinical demands, higher expectations for uptime, and a stronger emphasis on predictable lifecycle outcomes. As protocols push thermal limits and imaging becomes more operationally critical, stakeholders are moving beyond specification-based comparisons toward evidence of repeatable performance, service readiness, and supply continuity.Meanwhile, the 2025 U.S. tariff landscape reinforces the need for disciplined contracting and proactive risk management. Tariff exposure is not only a pricing issue; it influences lead times, inventory decisions, and supplier selection criteria. Organizations that integrate trade risk into their sourcing and service models are more likely to protect scanner availability and maintain budget stability.
Segmentation and regional insights point to a consistent conclusion: successful strategies are contextual. The best decisions align tube configuration and quality assurance rigor with clinical workload, service capability, and local procurement requirements. Companies that operationalize this alignment-through standardization, analytics-enabled maintenance, and outcome-based supplier governance-will be positioned to deliver reliable imaging capacity even as the landscape continues to evolve.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China = 5MHU CT Tubes Market
Companies Mentioned
The key companies profiled in this ≥ 5MHU CT Tubes market report include:- Canon Medical Systems Corporation
- Dunlee GmbH
- Fujifilm Holdings Corporation
- General Electric Company
- Hitachi, Ltd.
- IAE X‑Ray Tube, Inc.
- Konason Medical Technology Co., Ltd.
- Koninklijke Philips N.V.
- Kunshan YiYuan Medical Technology Co., Ltd.
- Neusoft Medical Systems Co., Ltd.
- Richardson Healthcare Ltd.
- Shimadzu Corporation
- Siemens Healthineers AG
- Strahlkraft Medical Technology Co., Ltd.
- United Imaging Healthcare Co., Ltd.
- Varex Imaging Corporation
- Zhuhai Rcan Vacuum Electron Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
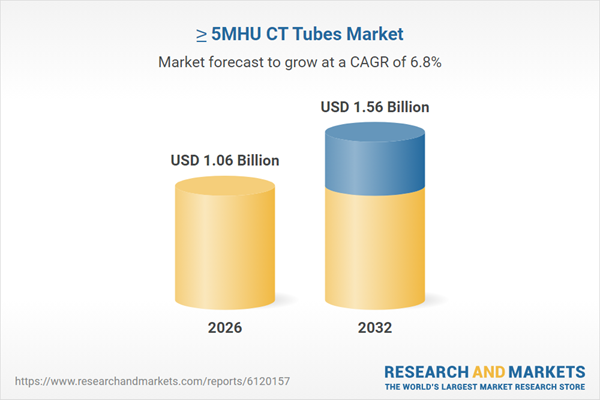
| Estimated Market Value ( USD | $ 1.06 Billion |
| Forecasted Market Value ( USD | $ 1.56 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |