Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction to ambulatory electrocardiography describing clinical value, technological evolution, and implications for modern care delivery
Ambulatory electrocardiography has moved from a niche diagnostic tool to a foundational component of contemporary cardiac care, bridging episodic clinic visits with continuous physiological insight. Clinicians rely on ambulatory ECG solutions to capture elusive arrhythmias, assess post‑procedural rhythm stability, and enable longitudinal patient management outside the hospital setting. Meanwhile, payers and health systems are increasingly focused on evidence that demonstrates clinical utility, care pathway optimization, and potential reductions in avoidable admissions.Technological innovations have expanded the modality repertoire available to clinicians and patients alike, enabling more discreet, comfortable, and extended monitoring. These advances have broadened diagnostic windows and improved patient adherence, facilitating earlier detection and more accurate characterization of arrhythmias and ischemic events. Equally important, integration of analytics and secure connectivity is reshaping how data flows from patient to clinician, enabling more timely interventions and richer population health analyses.
From a strategic standpoint, ambulatory electrocardiography now intersects with broader shifts in care delivery: decentralization of testing to home and community settings, elevated expectations for remote monitoring and telehealth, and the need for interoperable data streams that support both individual care decisions and system‑level quality initiatives. As a result, manufacturers, providers, and payers must align clinical validity, workflow compatibility, and reimbursement readiness to capture the full value of ambulatory ECG solutions.
Major technological, clinical, and commercial transformations that are redefining ambulatory electrocardiography adoption and stakeholder expectations
The landscape for ambulatory electrocardiography is being reshaped by several transformative shifts that affect clinical practice, procurement decisions, and patient engagement. First, miniaturization and ergonomic design have made sensors less obtrusive, encouraging longer wear times and thereby increasing diagnostic yield; the net effect is an expanded role for monitoring in both symptomatic and asymptomatic populations. Second, the integration of continuous and intermittent modalities with cloud‑based analytics has enabled automated flagging of clinically significant events, accelerating clinician response times and supporting scalable monitoring programs.Concurrently, advances in wireless connectivity and cybersecurity have made real‑time telemetry more dependable and compliant with evolving privacy standards. This has catalyzed adoption of real‑time mobile cardiac telemetry in ambulatory settings where rapid clinician notification alters management. Another important shift is the convergence of device data with electronic health records and remote patient monitoring platforms, which supports longitudinal care coordination and value‑based contracting. Furthermore, payer policies and reimbursement frameworks are gradually recognizing remote diagnostics as a core component of chronic disease management, incentivizing broader deployment.
Lastly, the entry of technology firms and digital health startups into the cardiac monitoring domain has intensified competition and innovation, prompting established medical device companies to pursue partnerships and platform strategies. As a result, stakeholders now evaluate ambulatory ECG offerings not only on signal fidelity, but also on software ecosystems, regulatory positioning, and capacity to integrate into hybrid care models that span home, clinic, and hospital environments.
How tariff adjustments in 2025 are reshaping sourcing, pricing, and strategic manufacturing decisions across the ambulatory electrocardiography value chain
The United States tariff landscape for 2025 introduces a material layer of commercial complexity for manufacturers and distributors of ambulatory electrocardiography devices and consumables. Tariff adjustments affect landed costs for devices sourced or assembled across diverse geographies, and they change procurement calculus for hospital systems and group purchasing organizations that manage capital and expendable budgets. In response, procurement teams are reassessing supplier mixes, total cost of ownership, and near‑term replacement cycles to preserve clinical continuity while containing costs.Manufacturers face the dual challenge of preserving margins and maintaining competitive pricing in markets where device commoditization is increasing. To mitigate tariff exposure, firms are evaluating alternative manufacturing footprints, nearshoring assembly operations, and negotiating longer‑term supply agreements to stabilize input prices. These strategic responses can also catalyze operational improvements, including enhanced quality control and reduced logistics lead times, but they require capital investment and regulatory coordination when manufacturing processes cross jurisdictions.
On the demand side, higher import costs can accelerate preference for domestically produced monitoring solutions or for service models that bundle monitoring hardware with cloud analytics and recurring revenue streams, thereby diluting per‑unit tariff effects. Health systems and clinics will increasingly emphasize procurement contracts that include warranty, service, and analytics to maximize value delivered under tighter budget constraints. Overall, tariffs in 2025 are prompting a reassessment of sourcing strategies, pricing models, and partnership structures across the ambulatory electrocardiography value chain.
Comprehensive segmentation insights revealing how product types, end users, channels, test types, modalities, and clinical applications shape ambulatory ECG adoption
Key segmentation insights illuminate how product design, clinical setting, distribution pathways, testing modality, form factor, and intended application interact to shape adoption of ambulatory electrocardiography solutions. Based on product type, the market encompasses ECG patches, event monitors, Holter monitors, and mobile cardiac telemetry; within ECG patches, the distinction between multi‑lead and single‑lead patch designs affects diagnostic granularity and clinical acceptance, while mobile cardiac telemetry divides into real‑time MCT and store‑and‑forward MCT, with each approach carrying different implications for latency, clinician workflow, and reimbursement. These product differences influence how clinicians select devices for specific diagnostic needs and patient preferences.When considering end users, ambulatory ECG is deployed across ambulatory surgery centers, clinics, diagnostic laboratories, homecare settings, and hospitals; device form factor, monitoring duration, and data integration requirements drive site‑specific preferences, with homecare settings and ambulatory centers emphasizing patient comfort and ease of use, while hospitals and diagnostic laboratories prioritize diagnostic robustness and workflow interoperability. Distribution channel dynamics further modulate access and adoption: direct sales relationships favor enterprise deployments and service contracts, whereas e‑commerce avenues-encompassing both B2B e‑commerce and direct‑to‑consumer models-support scalable retailing and patient‑initiated monitoring; hospital pharmacies and retail pharmacies provide established procurement routes for replaceable consumables and short‑term monitoring kits.
Test type segmentation between continuous monitoring and intermittent monitoring delineates clinical use cases; continuous monitoring is preferred for high‑risk patients requiring sustained surveillance and for detecting transient arrhythmias, while intermittent monitoring supports symptom‑correlated diagnostics and cost‑sensitive applications. Modality considerations separate wearable and wired systems; wearables further split into patch and strap‑based formats, offering differing tradeoffs in skin adherence, lead configuration, and patient mobility, while wired solutions such as cable‑based systems maintain advantages in signal stability for certain clinical protocols. Application segmentation across arrhythmia detection, general cardiac screening, ischemic monitoring, and remote patient monitoring informs both clinical pathway design and commercial positioning, with remote patient monitoring in particular acting as a bridge between device capability and chronic disease management programs. Taken together, these segmentation layers reveal that successful offerings are those that align technical performance with the operational realities of target end users and distribution channels, while delivering the specific test type and modality appropriate to the intended clinical application.
Regional assessment of ambulatory ECG adoption trends highlighting distinct clinical, regulatory, and commercial dynamics across global territories
Regional dynamics influence technology preference, procurement behavior, and regulatory priorities in ambulatory electrocardiography across major global territories. In the Americas, clinical adoption is driven by an emphasis on data‑driven care, payer engagements for remote monitoring, and well‑established hospital and outpatient infrastructures that facilitate rapid deployment of both enterprise and consumer monitoring solutions. Healthcare systems in this region often prioritize interoperability with electronic records and demonstrate a readiness to pilot integrated monitoring programs that link diagnostics with care pathways.In Europe, Middle East & Africa, the regulatory environment and heterogeneous healthcare financing models create differentiated opportunities and barriers: some markets exhibit robust reimbursement mechanisms for remote diagnostics, enabling scalable programs, while others require localized clinical validation and tailored service models. Infrastructure variability, particularly in parts of Africa and the Middle East, shapes deployment strategies, favoring low‑complexity, durable devices and solutions that can operate with constrained connectivity. Local regulatory harmonization efforts and pan‑regional procurement initiatives are increasingly important for firms seeking broader access.
Asia‑Pacific presents a mix of high‑volume, innovation‑oriented markets alongside regions focused on expanding basic cardiac services. Rapid urbanization, rising chronic disease burdens, and investments in digital health infrastructure support accelerated uptake of wearable and cloud‑enabled monitoring solutions in several countries, while supply chain localization and strategic partnerships with regional distributors are critical enablers for market entry. Across all regions, regulators and health systems are converging on priorities of clinical evidence, data security, and cost‑effective care delivery, which in turn guide adoption and commercialization strategies.
Competitive landscape analysis showing how device makers, analytics platforms, and service integrators are reshaping commercial and clinical rivalry
Competitive dynamics in ambulatory electrocardiography are defined by a mix of specialized device manufacturers, software platform providers, service integrators, and emerging health technology entrants. Established medical device companies continue to leverage clinical credibility, regulatory approvals, and broad distribution networks to defend core positions, while software‑first firms push capabilities in cloud analytics, machine learning, and patient engagement to differentiate user experience and downstream value. Service providers that bundle monitoring hardware with analytics, clinician triage, and reimbursement support are shaping new value propositions that appeal to health systems seeking turnkey remote monitoring programs.Partnerships and strategic collaborations are increasingly common, as device manufacturers seek to augment hardware with best‑in‑class analytics or to extend market reach through channel partners. Technology entrants often create competitive pressure on pricing and user expectations but can also accelerate category expansion by introducing consumer‑friendly interfaces and direct‑to‑patient distribution. Regulatory clearance pathways and evidence generation remain critical competitive levers; companies that invest in clinical validation and real‑world outcome studies gain traction with clinicians and payers. Furthermore, consolidation through selective acquisitions is occurring as larger players aim to secure software capabilities, expand geographic footprints, and obtain curated patient data assets that support longitudinal care models. Ultimately, competitive success hinges on the ability to combine reliable physiological sensing with seamless data flow, validated algorithms, and sustainable service economics.
Actionable strategic recommendations for industry leaders to align product design, commercial models, and evidence generation to accelerate adoption
Industry leaders should pursue a three‑pronged strategy that aligns product design, commercial models, and evidence generation to secure sustainable adoption. First, prioritize modular product roadmaps that enable configuration for distinct clinical needs: multi‑lead and single‑lead patch options, real‑time or store‑and‑forward telemetry selections, and wearable versus wired formats should be designed to minimize manufacturing complexity while maximizing clinical fit. This approach facilitates targeted value propositions for ambulatory surgery centers, clinics, hospitals, diagnostic laboratories, and homecare settings without sacrificing economies of scale.Second, adapt distribution and commercial models to evolving procurement channels by strengthening direct sales relationships for enterprise customers while developing robust e‑commerce capabilities that address both B2B and direct‑to‑consumer opportunities. Include service‑based pricing and subscription structures that bundle analytics, clinical triage, and device replacement to mitigate tariff or unit price sensitivity and to cultivate recurring revenue streams. Align go‑to‑market efforts with hospital pharmacies and retail pharmacy partnerships to enhance point‑of‑care availability for short‑term monitoring.
Third, accelerate clinical and health economic evidence generation focused on the specific applications of ambulatory ECG-arrhythmia detection, general cardiac screening, ischemic monitoring, and remote patient monitoring-and ensure interoperability with electronic health records and remote monitoring platforms. This evidence will support payer discussions and facilitate integrations that make monitoring data actionable within care pathways. Finally, invest in supply chain flexibility-nearshoring options, strategic inventory buffers, and diversified component sourcing-to reduce exposure to tariff and logistics disruptions while preserving service levels for customers.
Rigorous multi‑method research methodology combining expert interviews, product mapping, and validation to produce operationally relevant insights
The research methodology underpinning this analysis combines qualitative expert interviews, targeted stakeholder validation, product mapping, and secondary literature synthesis to ensure balanced and actionable conclusions. Primary qualitative inputs were obtained from clinicians, procurement directors, and technology leaders to validate real‑world workflows, device performance requirements, and adoption barriers. These insights were cross‑referenced with product specifications, regulatory filings, and publicly available clinical literature to triangulate claims about modality capabilities, wearability tradeoffs, and typical use cases.Commercial channel dynamics and tariff implications were assessed through consultations with supply chain specialists, distributors, and market access advisors, enabling a grounded view of pricing sensitivities and sourcing strategies. Comparative analysis of device form factors and test types relied on technical specifications and peer‑reviewed studies to evaluate diagnostic robustness and patient tolerability. Throughout the methodology, emphasis was placed on pragmatic validation: scenarios and recommendations were stress‑tested against operational constraints such as clinician workflow, connectivity variability, and payer policy differences. This multi‑method approach yields insights that are both evidence‑based and operationally relevant for stakeholders seeking to implement or scale ambulatory ECG programs.
A conclusive synthesis emphasizing strategic priorities and the integrated actions required to lead in ambulatory cardiac monitoring innovation
In conclusion, ambulatory electrocardiography occupies a pivotal role in contemporary cardiovascular care by enabling extended, patient‑centric monitoring that informs timely clinical interventions and supports chronic disease management. Technological advances in patch design, telemetry, and analytics have enhanced diagnostic capture and clinician workflows, while evolving commercial models and tariff considerations are reshaping sourcing and pricing strategies. Effective market participation requires alignment of product capabilities with end‑user needs, thoughtful channel strategies that encompass both enterprise and consumer pathways, and robust clinical evidence that speaks to specific applications such as arrhythmia detection and remote patient monitoring.Moving forward, stakeholders who integrate flexible manufacturing approaches, invest in interoperability and validated analytics, and adopt bundled service models will be best positioned to navigate regulatory, reimbursement, and supply chain uncertainties. The confluence of device innovation, software‑driven insights, and new care delivery models presents a significant opportunity to expand the clinical reach of ambulatory ECG while improving patient outcomes and operational efficiency. Strategic action now will determine which organizations lead the next phase of ambulatory cardiac monitoring.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Ambulatory Electrocardiography Market
Companies Mentioned
The key companies profiled in this Ambulatory Electrocardiography market report include:- AliveCor, Inc.
- Bionet Co., Ltd
- BioTelemetry, Inc.
- Cardiac Insight Inc.
- Fukuda Denshi Co., Ltd.
- General Electric Company
- iRhythm Technologies, Inc.
- Koninklijke Philips N.V.
- Medtronic plc
- Mindray Medical International Limited
- Nihon Kohden Corporation
- OSI Systems, Inc.
- Schiller AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
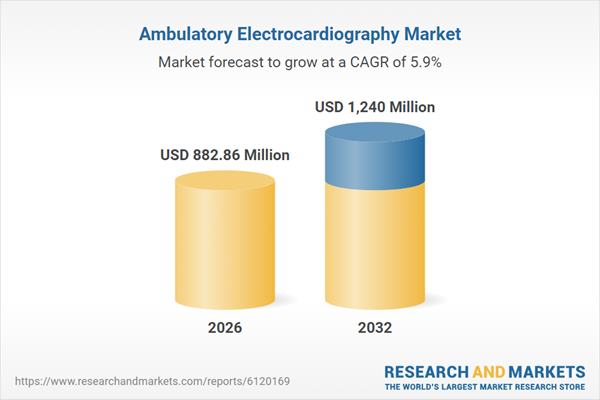
| Estimated Market Value ( USD | $ 882.86 Million |
| Forecasted Market Value ( USD | $ 1240 Million |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |