Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive orientation to the converging technological, regulatory, commercial, and sustainability forces reshaping microbial testing consumables
The microbial testing consumables landscape is characterized by rapid technological change, evolving regulatory expectations, and heightened sensitivity to supply continuity. Laboratories and industrial testing facilities are balancing traditional culture-based workflows with an accelerating adoption of molecular and immunoassay-based techniques, while end users demand higher throughput, traceability, and ease of use. As a result, consumables that enable faster turnaround, reduce contamination risk, and integrate with digital laboratory ecosystems are moving from optional to essential in many settings.Across clinical, pharmaceutical, food and beverage, environmental, and water testing contexts, purchasing decisions are increasingly influenced by product reliability, validated performance under regulatory regimes, and supplier service capabilities. In parallel, sustainability considerations and single-use plastic debates are prompting renewed attention to material selection and disposal pathways. Taken together, these forces are reshaping product development priorities and procurement strategies for manufacturers and buyers alike.
Moreover, the competitive environment is driven by innovation in assay chemistry, membrane technologies, and sampling ergonomics. New entrants and established suppliers are investing in product lines that reduce hands-on time and support decentralized testing models. Consequently, executives must navigate a complex set of trade-offs between legacy systems and next-generation platforms, ensuring that choices made today preserve analytical integrity while enabling operational scalability.
Decisive technological, regulatory, and sustainability inflection points are reshaping product development, procurement priorities, and supplier strategies in consumables
The landscape for microbial testing consumables is undergoing transformative shifts driven by convergent technological advances, regulatory tightening, and changing end-user expectations. First, molecular diagnostics and high-throughput sequencing approaches are migrating from specialist laboratories into routine testing environments, creating demand for molecular reagents, PCR and qPCR kits, and sequencing-compatible consumables that meet stricter contamination control standards. At the same time, culture media formulations are evolving with chromogenic and selective agars offering faster presumptive identification, which reduces downstream testing burden and accelerates decision-making in quality control workflows.Second, automation and digital integration are redefining how consumables are designed and procured. Automated liquid handling platforms, integrated environmental monitoring systems, and data management solutions demand consumables with consistent performance, barcoding, and traceability features. Consequently, design priorities now include lot-to-lot consistency, compatibility with robotics, and packaging that supports sterile transfer in automated settings.
Third, regulatory scrutiny and accreditation expectations have intensified across clinical and industrial applications, prompting suppliers to strengthen validation datasets, provide robust certificates of analysis, and support customer audits with comprehensive documentation. Finally, sustainability pressures have accelerated interest in alternative materials and reusable components where feasible, even as single-use disposables remain necessary for contamination control. Together, these shifts are driving a rebalancing of R&D investment, supply chain strategies, and commercial go-to-market approaches that prioritize flexibility, compliance, and operational continuity.
Assessment of how recent United States tariff adjustments have structurally altered sourcing, production footprints, and procurement risk management across consumables supply chains
Recent tariff developments originating from fiscal and trade policy shifts have introduced additional complexity into global procurement decisions for microbial testing consumables. Increased duties and altered classification of laboratory consumables can directly influence landed costs and supplier selection, while secondary effects ripple through contract manufacturing, component availability, and logistics planning. Importantly, tariffs amplify the effects of existing supply-chain fragilities, making timely access to critical items such as membrane filters, reagents, and single-use disposables more uncertain for laboratories that rely on cross-border sourcing.In response, many organizations recalibrated sourcing strategies by diversifying supplier bases, increasing safety stock for critical SKUs, and, in some cases, shifting to regional suppliers to reduce exposure to tariff-related cost volatility. Simultaneously, contract terms have been renegotiated to share risk, with buyers seeking clauses that address duty pass-throughs, lead-time assurances, and contingency production plans. For manufacturers, tariffs have incentivized localization of certain production stages, including final assembly and packaging, to mitigate the impact of import duties on finished goods.
From an operational perspective, procurement teams are placing greater emphasis on tariff classification expertise and harmonized system codes to avoid unintended reclassifications that could trigger additional duties. Strategic sourcing now frequently incorporates scenario planning that models tariff permutations and their potential effect on supplier economics and delivery performance. As such, tariff-related dynamics are not a one-time cost issue but a structural factor shaping supplier selection, inventory strategy, and long-term manufacturing footprints.
Multi-dimensional segmentation insights that reveal nuanced product, end-user, technological, and application-specific drivers shaping procurement and innovation
Analyzing consumables through multiple segmentation lenses reveals differentiated demand drivers that inform product development and commercial prioritization. When considering product type, the portfolio spans culture media, disposables and accessories, filtration apparatus, reagents and assay kits, and sampling devices. Within culture media, distinctions between agar media, broth media, and chromogenic media matter profoundly for laboratory workflows; agar media itself subdivides into formulations such as blood agar, MacConkey agar, nutrient agar, and Sabouraud Dextrose agar, each tailored to specific organism groups and quality control uses. Disposables and accessories encapsulate items like culture tubes, petri dishes, and pipette tips and plates, and within pipette consumables there is a meaningful split between filtered pipette tips and standard pipette tips that affects contamination control strategies.Filtration apparatus covers the spectrum from filter holders and membrane filters to vacuum pumps, with membrane filters further differentiated into cellulose nitrate and polycarbonate variants that influence recovery rates and downstream analyses. Reagents and assay kits include biochemical reagents, immunoassay kits, and molecular reagents, where molecular reagents branch into PCR kits, qPCR kits, and sequencing kits that support different levels of analytical resolution. Sampling devices complete the product segmentation with air samplers, sponges, and swabs, and swab selection ranges from flocked swabs to sterile cotton swabs, each impacting sample integrity and ease of use.
From an end-user perspective, purchasing drivers vary across clinical laboratories, cosmetic manufacturers, environmental testing services, food and beverage operations, pharmaceutical and biotechnology firms, and water treatment facilities. The food and beverage vertical further subdivides into bakery and confectionery, beverage processing, and dairy and meat processing, each with distinct contamination risk profiles and sampling protocols. The pharmaceutical and biotechnology arena includes biologics manufacturing, generics manufacturing, and sterile drug manufacturing, where sterile consumables and validated reagents are non-negotiable for regulatory compliance.
Technology segmentation underscores divergent adoption pathways; chromatographic methods, culture-based systems, immunoassays, and molecular diagnostics each present distinct consumable needs. Within culture-based systems, methods like membrane filtration, most probable number, and plate count have unique consumable footprints. Molecular diagnostics incorporate techniques such as LAMP, next generation sequencing, and PCR-based methods, each dictating specific reagent purity and handling requirements. Finally, application-based segmentation-spanning air monitoring, general quality control, pathogen detection, sterility testing, and water quality monitoring-reveals use-case driven preferences, particularly in pathogen detection where workflows for E. coli, Listeria, and Salmonella demand targeted consumables and validated protocols.
Together, these segmentation lenses highlight where product innovation and tailored commercial approaches are most likely to generate adoption. They show that a one-size-fits-all approach is untenable; instead, manufacturers and procurement teams must align product design, validation evidence, and service capabilities with the nuanced needs of specific subsegments to deliver practical value and reduce operational risk.
How differentiated regional procurement behaviors and regulatory landscapes across the Americas Europe Middle East & Africa and Asia-Pacific influence supplier competitiveness and demand
Regional dynamics exert a strong influence on procurement patterns, regulatory requirements, and supplier strategies across the globe. In the Americas, demand is shaped by a large and diversified end-user base, with significant emphasis on clinical diagnostics, food and beverage quality control, and pharmaceutical manufacturing. Buyers in this region increasingly prioritize validated product performance, supplier responsiveness, and domestic availability to mitigate shipping delays and tariff exposure, and as a result regional manufacturing and distribution nodes are receiving greater strategic focus.Shifts in regulatory frameworks and harmonization efforts in Europe, Middle East & Africa create a complex environment in which compliance documentation, batch-level traceability, and support for certification audits become key differentiators for suppliers. Laboratories in this region often require multi-lingual support materials and localized regulatory expertise, and suppliers that can provide comprehensive technical support and robust documentation find an advantage. Meanwhile, emerging markets within the broader region are developing testing infrastructure that elevates demand for reliable, easy-to-use consumables and training support.
Asia-Pacific presents a heterogeneous mix of rapid adoption of molecular diagnostics, expansion of biologics manufacturing, and growing food safety enforcement. This region’s scale and manufacturing capacity make it both a major sourcing hub and a large customer base, but buyers increasingly seek suppliers that can guarantee consistent quality across production sites. Consequently, supply chain strategies that combine local inventory buffers with strategic regional partners are common, enabling faster response times while balancing cost and compliance considerations. Across all regions, suppliers that can demonstrate localized service, validated product performance, and flexible logistics arrangements maintain a competitive edge.
Strategic behaviors and competitive differentiators among suppliers that drive innovation adoption quality assurance and supply reliability in consumables
Competitive dynamics among manufacturers and distributors of microbial testing consumables center on product innovation, regulatory support, and operational excellence. Leading firms differentiate through investments in validated reagents, automated-compatibility of disposables, and expanded documentation to support audits and regulatory filings. In addition, strategic partnerships with instrument vendors and laboratory automation providers are a recurring theme, enabling integrated solutions that simplify procurement and reduce total cost of ownership for end users.Mergers, acquisitions, and strategic alliances are frequently used to acquire niche technologies or to expand geographic reach, while contract manufacturing relationships provide scalability for companies seeking to manage variable demand without material capital expenditure. Supply reliability and quality management systems remain primary competitive levers: firms that demonstrate consistent lot-to-lot performance, expedited technical support, and rapid supply restoration during disruptions capture greater loyalty from sensitive end users such as sterile drug manufacturers and clinical testing laboratories.
Innovation is not solely technological but also operational. Firms that offer accessible training, robust validation protocols, and data integration capabilities with laboratory information management systems strengthen customer retention. At the same time, smaller niche suppliers that focus on specialized reagents, unique membrane chemistries, or ergonomically designed sampling devices can command premium positioning in targeted verticals. Collectively, these behaviors create a landscape in which collaboration, demonstrated quality, and an ability to scale service delivery determine long-term competitive advantage.
A practical set of priority actions industry leaders must implement to strengthen supply resilience compliance and product-led differentiation in consumables
Industry leaders should prioritize a set of actionable measures to maintain resilience, capture growth, and meet evolving customer expectations. First, diversify sourcing strategies to reduce geographic concentration risk by qualifying multiple suppliers and exploring nearshoring for critical components, while maintaining agreements that protect access during peak demand. Second, invest in automation-compatible product designs and validation datasets that demonstrate performance in both manual and automated workflows, as this compatibility increasingly influences procurement decisions.Third, strengthen regulatory and quality documentation capabilities to support customer audits and to expedite approvals, particularly for products used in pharmaceutical and clinical contexts. Fourth, implement advanced inventory analytics and scenario-based planning that incorporate tariff contingencies and logistics disruptions, thereby enabling procurement teams to make informed trade-offs between cost and service levels. Fifth, accelerate sustainability initiatives where they do not compromise analytical integrity by exploring recyclable packaging, validated reduction in plastic usage, and life-cycle assessments that resonate with corporate ESG commitments.
Finally, cultivate strategic partnerships with instrument vendors, laboratory automation providers, and end-user training organizations to deliver bundled solutions that lower the operational burden for customers. By taking these steps, leaders can enhance supply continuity, foster customer loyalty, and position their organizations to respond quickly to technological and regulatory shifts.
A transparent and robust mixed-methods research methodology combining interviews literature review and expert validation to produce actionable consumables insights
The research underpinning these insights combined primary and secondary evidence sources with iterative expert validation to ensure reliability and relevance. Primary research included structured interviews with laboratory directors, procurement leaders, quality assurance professionals, and R&D managers across clinical, pharmaceutical, food and beverage, and environmental testing organizations. These discussions explored procurement criteria, product performance drivers, supplier selection rationales, and the operational impact of recent policy changes.Secondary research comprised a systematic review of public regulatory guidance, technical white papers, peer-reviewed literature on assay performance, and product literature from suppliers to map technology trends and product specifications. Data triangulation was used to reconcile differences between supplier claims and end-user experiences, and to surface consistent performance attributes valued across segments. In addition, case studies of supply disruptions and tariff impacts were analyzed to extract best practices in contingency planning and supplier negotiation.
Finally, findings were validated through expert panels that included laboratory scientists, regulatory consultants, and supply-chain strategists to ensure that conclusions were actionable and grounded in operational realities. Throughout the process, emphasis was placed on transparency in methodology, reproducibility of key findings, and clear linkage between evidence and recommended actions.
Synthesis of the report’s core findings highlighting strategic implications and the operational priorities that will determine success in consumables markets
In summary, the microbial testing consumables sector is at an inflection point where technological innovation, regulatory rigor, and supply-chain realities intersect. Laboratories and industrial users are increasingly seeking consumables that not only meet analytical performance criteria but also support automation, traceability, and sustainability objectives. Tariff dynamics and geopolitical influences have reinforced the importance of diversified sourcing and regional supply strategies, while segmentation analysis highlights the need for product portfolios that reflect the specific requirements of distinct end users, technologies, and applications.Competitive advantage will accrue to suppliers that combine validated product performance, robust quality systems, and responsive commercial models that include training and integration support. For buyers, the imperative is to align procurement practices with risk-aware inventory strategies, invest in compatibility with evolving technologies, and demand the documentation and support necessary to maintain compliance. Moving forward, the ability to translate technical innovation into reliable, auditable, and service-backed consumables will determine who captures value in this evolving ecosystem.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Microbial Testing Consumables Market
Companies Mentioned
The key companies profiled in this Microbial Testing Consumables market report include:- 3M Company
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- bioMérieux SA
- Charles River Laboratories International, Inc.
- Danaher Corporation
- Eurofins
- Lonza Group Ltd.
- Merck KGaA
- Neogen Corporation
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
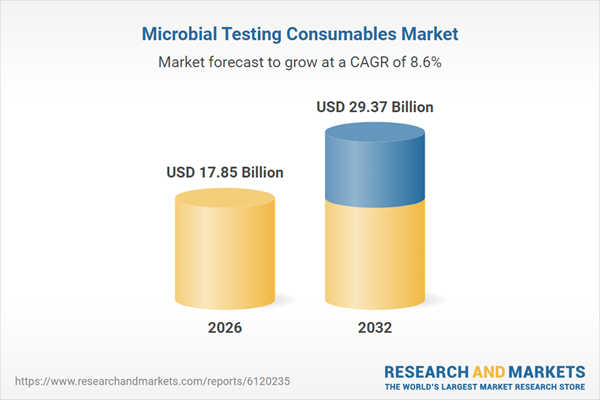
| Estimated Market Value ( USD | $ 17.85 Billion |
| Forecasted Market Value ( USD | $ 29.37 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |