Speak directly to the analyst to clarify any post sales queries you may have.
Rapid-read biological indicators are reshaping sterility assurance by combining faster actionable results with rigorous validation and compliance discipline
Rapid-read biological indicators have become a critical bridge between uncompromising sterility assurance and the operational demand for faster turnaround times. As healthcare systems, medical device manufacturers, and life science laboratories intensify their focus on patient safety and contamination control, the limitations of traditional incubation windows are increasingly visible in day-to-day workflows. Rapid-read formats respond directly to that pain point by providing earlier readouts that support quicker quarantine release decisions while preserving the fundamental purpose of a biological indicator: verifying that a sterilization process reliably inactivates highly resistant microorganisms.In parallel, the sector is being shaped by tighter quality management expectations, more frequent audits, and the heightened scrutiny applied to sterilization validation and routine monitoring. Facilities are being asked to demonstrate not only that sterilization cycles pass, but that controls are robust, deviations are triaged quickly, and documentation is consistent across sites. Rapid-read indicators, when properly integrated with incubators, readers, and digital records, fit naturally into these expectations because they compress the time between cycle completion and actionable confirmation.
At the same time, adoption is not simply a matter of replacing one consumable with another. Decision-makers must weigh compatibility with sterilization modalities, the practicality of incubation and readout in the intended environment, the credibility of supplier validation data, and the total workflow impact from loading to record retention. Consequently, this market is best understood as a system of products, processes, and compliance obligations rather than a single component category, with value realized only when speed, reliability, and traceability advance together.
Industry change is accelerating as sterility assurance shifts toward digital traceability, workflow integration, and resilient supply built for audit-ready speed
A central shift in the landscape is the move from time-based assurance to information-based assurance. Facilities increasingly expect biological monitoring to deliver rapid, decision-grade information that can be logged, trended, and audited with minimal manual intervention. This has accelerated the integration of rapid-read indicators with dedicated readers and incubation platforms that support standardized handling, automated result capture, and clearer exception management when a cycle result requires escalation.Another transformative change is the growing emphasis on end-to-end traceability across the sterilization ecosystem. Quality leaders are pushing for harmonized procedures that connect cycle parameters, load configuration, chemical indicators, biological indicators, and corrective actions into a coherent record. In response, manufacturers are improving label design, lot traceability, and device compatibility while also supporting digital workflows that reduce transcription errors and shorten deviation investigations.
Material and design innovation is also advancing under practical constraints. Product developers are working to balance faster readout with storage stability, ease of use under PPE and cleanroom conditions, and robust performance across variable loads. As sterilization cycles diversify and packaging configurations evolve, customers are demanding evidence that rapid-read performance is dependable under real-world stresses, not only in idealized validation settings.
Finally, supply resilience has shifted from a procurement concern to a quality imperative. Sterility assurance programs cannot tolerate interruptions, so buyers now evaluate second-sourcing strategies, regional manufacturing footprints, and logistics reliability more rigorously. This change favors suppliers that can document continuity planning and sustain consistent quality across production lots, especially when rapid-read indicators become embedded as standard operating practice rather than occasional verification tools.
United States tariffs in 2025 are elevating trade-aware sourcing into a sterility assurance priority by reshaping costs, continuity planning, and qualification needs
The 2025 tariff environment in the United States adds a layer of complexity to procurement and supplier strategy for rapid-read biological indicators and their associated equipment. Because these programs rely on steady replenishment of consumables and calibrated devices, even modest cost shocks or customs delays can ripple into sterilizer utilization, release timing, and deviation risk. Tariff exposure can affect not only finished indicators but also critical inputs such as plastics, specialty packaging, enzymes or reagents used in rapid detection systems, and electronic components used in readers.In practice, tariffs tend to influence buying behavior in three interconnected ways. First, they increase the perceived value of supply continuity, pushing organizations to qualify alternate SKUs, dual-source across regions, and tighten inventory policies for essential monitoring products. Second, they amplify total cost-of-ownership conversations, where price is evaluated alongside documentation support, delivery performance, lot-to-lot consistency, and the operational cost of slower release decisions if substitutes revert to longer incubation windows. Third, they intensify scrutiny of country-of-origin declarations and the upstream supply chain, particularly for regulated environments where documentation integrity is central to audit outcomes.
Manufacturers and distributors are responding by reassessing manufacturing location strategies, adjusting logistics pathways, and strengthening customs documentation processes to reduce clearance friction. Some are also emphasizing modularity in equipment and accessories to reduce dependence on tariff-sensitive parts. For buyers, the strategic implication is clear: tariff impacts should be handled as a sterility assurance risk factor, not merely as a budget line item, because delayed availability or forced substitutions can undermine standardized monitoring protocols.
Over the next procurement cycles, the most resilient programs will be those that pair technical qualification with trade-aware sourcing. That means validating equivalents before disruption occurs, aligning quality and purchasing teams on acceptance criteria, and building contractual expectations around lead times, notification requirements for material changes, and documentation support. When tariffs change the economics of supply, preparedness becomes the differentiator between maintaining a stable sterility assurance cadence and absorbing avoidable operational turbulence.
Segmentation reveals rapid-read indicator demand is shaped by sterilization modality, product configuration, end-use workflow maturity, and purchasing pathways
Segmentation in rapid-read biological indicators highlights how performance expectations and purchasing criteria vary depending on modality, workflow setting, and compliance posture. Across product type distinctions, self-contained rapid-read indicators are often selected where standardized handling and reduced contamination risk matter most, while configurations that emphasize flexible placement and compatibility can be favored in complex load geometries. The choice is frequently shaped by how quickly a facility needs a result to support release decisions and how consistently staff can execute incubation and readout steps in a controlled manner.Differences across sterilization methods remain one of the most decisive segmentation drivers. Rapid-read solutions aligned to steam sterilization are commonly evaluated on robustness under high moisture and temperature conditions, as well as clarity of readout and incubation practicality. In contrast, solutions designed for low-temperature modalities such as ethylene oxide and hydrogen peroxide must address distinct process dynamics, including material compatibility and the operational need to verify lethality without extending already complex aeration and turnover constraints. Where facilities operate multiple modalities, standardization efforts often focus on minimizing training variation while preserving modality-specific performance.
End-user context further differentiates demand. Hospitals and ambulatory care centers prioritize operational simplicity, fast turnaround, and documentation that supports infection prevention and accreditation. Medical device and pharmaceutical manufacturers, by comparison, tend to emphasize validation rigor, lot traceability, and integration with quality systems that govern batch release and deviation investigations. Laboratories and research environments often sit between these priorities, balancing speed with repeatability and the need to maintain consistent controls across varied protocols.
Procurement pathways also shape how value is captured. Direct purchasing may be preferred when technical support, training, and validation documentation are central to internal qualification, while distributors can add value through consolidated logistics, faster replenishment, and bundling with complementary sterilization consumables. Additionally, segmentation by application clarifies why some organizations concentrate rapid-read indicators in routine monitoring while others deploy them more heavily in qualification, requalification, and load-specific challenge studies. Across these segments, the unifying insight is that rapid-read adoption succeeds when the indicator is matched to the modality, the operational maturity of the site, and the strength of the documentation ecosystem supporting the sterility assurance program.
Regional patterns show adoption varies with healthcare maturity, manufacturing concentration, and supply reliability across the Americas, EMEA, and Asia-Pacific
Regional dynamics underscore how regulatory emphasis, healthcare infrastructure, and manufacturing footprints influence adoption patterns for rapid-read biological indicators. In the Americas, demand is closely tied to mature sterilization practices in hospitals and strong quality expectations in medical device and life science manufacturing. The region’s emphasis on audit readiness and consistent documentation drives interest in solutions that streamline records and shorten the time between cycle completion and disposition decisions.Across Europe, the Middle East, and Africa, adoption is shaped by a blend of established healthcare systems, cross-border supply considerations, and varied levels of sterilization infrastructure maturity. Facilities operating under stringent quality frameworks often prioritize harmonized procedures and multi-site standardization, which elevates the importance of product consistency and strong supplier documentation. In emerging areas within this broad region, training, service availability, and dependable distribution networks can be as influential as product performance in determining successful deployment.
In the Asia-Pacific region, growth in healthcare capacity and continued expansion of manufacturing ecosystems are key drivers of demand for reliable sterility assurance tools that can scale. Organizations frequently evaluate rapid-read solutions through the lens of throughput and operational efficiency, especially where high-volume sterilization operations support medical device production or large hospital networks. At the same time, procurement decisions may be influenced by localization strategies, the availability of technical support, and the ability of suppliers to ensure consistent lead times across diverse geographies.
Across all regions, a common theme is the rising importance of resilience. Buyers increasingly value suppliers that can support multi-country operations with consistent quality, clear traceability, and training resources that help standardize interpretation and escalation protocols. As a result, regional strategies are converging around the same objective: reduce uncertainty in sterility assurance by combining rapid results with dependable supply and audit-ready evidence.
Company differentiation is shifting toward validated end-to-end workflows, modality breadth, dependable service, and data integrity that withstands audits
Competitive positioning in rapid-read biological indicators is increasingly defined by the ability to deliver a complete, validated workflow rather than a standalone consumable. Leading companies differentiate through the credibility of their performance validation, the usability of their incubation and reader platforms, and the practical support they provide for implementation. This includes training that reduces operator variability, documentation packages that accelerate internal qualification, and clear guidance for integrating rapid-read results into deviation handling and release decision logic.Another axis of differentiation is portfolio breadth across sterilization modalities and use cases. Suppliers that can support steam as well as low-temperature processes are better positioned for customers seeking standardization across departments and sites. Portfolio breadth also matters in how well companies support both routine monitoring and qualification activities, where the demands for documentation depth and protocol flexibility can diverge.
Service capability and post-sale support are becoming more visible competitive levers. Facilities want assurance that readers and incubation devices remain calibrated, that consumables are consistently available, and that any product change is communicated with sufficient lead time to protect validated states. Companies that offer strong technical service networks, reliable logistics, and disciplined change control practices build trust in regulated environments where surprises translate into compliance risk.
Finally, innovation is increasingly oriented toward data integrity and operational integration. Suppliers are investing in clearer result interpretation, more consistent readout timing, and features that reduce transcription and handling errors. As customers pursue tighter quality governance, companies that enable standardized digital records and simplified audits-without adding workflow friction-tend to earn deeper adoption and longer retention.
Leaders can turn rapid-read adoption into measurable operational control by standardizing workflows, qualifying rigorously, and hardening supply and data governance
Industry leaders can strengthen their sterility assurance programs by treating rapid-read biological indicators as part of a controlled system, not a swap-in consumable. Start by mapping the current-state workflow from cycle completion to load release, identifying where delays, manual transcription, or unclear escalation rules create risk. Then align rapid-read deployment with the points of greatest operational impact, such as high-throughput sterilizers, critical surgical instrument flows, or manufacturing steps where release timing drives downstream scheduling.Next, prioritize qualification discipline and standardization. Establish clear acceptance criteria, handling procedures, and documentation requirements before expanding across sites. Where multiple sterilization modalities are used, develop modality-specific protocols while keeping training and recordkeeping as consistent as possible. This approach reduces operator variability and improves audit defensibility, especially when staff rotate across shifts or departments.
Supply resilience should be built into sourcing decisions. Qualify alternate products where feasible, evaluate supplier change control practices, and negotiate expectations for notification of material or process changes. In parallel, review inventory policies to ensure continuity without creating waste, and assess whether regional warehousing or distributor partnerships reduce lead-time volatility.
Finally, invest in data governance. Ensure rapid-read results are captured in a manner consistent with quality system expectations, with traceable linkage to sterilizer cycles, load identifiers, and corrective actions when needed. When data integrity is treated as a design requirement rather than an afterthought, rapid-read indicators can deliver their full value: faster decisions, fewer ambiguous outcomes, and a sterility assurance narrative that holds up under scrutiny.
Methodology blends stakeholder interviews, standards-led secondary review, and triangulated validation to produce practical, audit-relevant market insight
The research methodology for this report combines structured primary engagement with rigorous secondary analysis to produce decision-ready insight into rapid-read biological indicators. Primary inputs include interviews and discussions with stakeholders across sterilization operations, quality assurance, infection prevention, procurement, and supplier organizations, with attention to practical implementation realities such as training burden, failure investigation workflows, and audit documentation needs.Secondary research synthesizes publicly available regulatory guidance, standards frameworks, technical literature, product documentation, and company disclosures relevant to biological monitoring and rapid-read technologies. This layer is used to validate terminology, clarify modality-specific considerations, and ensure that the discussion reflects current compliance expectations and technology directions without relying on speculative claims.
Analytical techniques emphasize triangulation and consistency checks across inputs. Insights are cross-validated by comparing perspectives from different roles in the value chain, reconciling operational claims with documented requirements, and stress-testing conclusions against observed procurement and implementation constraints. The goal is to present a balanced view of how rapid-read indicators are selected, qualified, and used in real environments.
Throughout the process, the research is structured to remain practical for decision-makers. Findings are framed around workflow impact, risk management, supplier capability, and implementation prerequisites so that readers can translate insights into procurement specifications, qualification plans, and operational improvements.
Rapid-read biological indicators deliver their strongest value when speed, modality fit, and documentation integrity align with enterprise quality expectations
Rapid-read biological indicators are increasingly central to modern sterilization assurance because they compress decision cycles while supporting robust compliance. As organizations face higher throughput expectations and tighter audit scrutiny, the ability to obtain earlier biological confirmation becomes a meaningful operational advantage-provided that implementation is controlled, validated, and supported by strong documentation practices.The landscape is evolving toward integrated workflows that prioritize traceability, reduce manual handling, and strengthen data integrity. At the same time, external pressures such as the 2025 tariff environment are pushing buyers to treat sourcing stability as part of quality risk management. These forces collectively favor solutions that combine modality-appropriate performance with dependable supply, service, and change control.
Ultimately, successful adoption depends on fit: matching the indicator and its supporting ecosystem to the sterilization modality, the end-user workflow, and the organization’s quality maturity. When that alignment is achieved, rapid-read biological indicators can help organizations improve turnaround times, reduce uncertainty, and strengthen confidence in sterility assurance decisions across routine operations and high-stakes applications.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Rapid-Read Biological Indicators Market
Companies Mentioned
The key companies profiled in this Rapid-Read Biological Indicators market report include:- 3M Company
- Andersen Products
- Crosstex International, Inc.
- Ecolab Inc.
- Getinge AB
- Healthmark Industries Company, Inc.
- Liofilchem S.r.l.
- Matachana Group
- MELAG Medizin-Technik GmbH & Co. KG
- Mesa Laboratories, Inc.
- Microbiologics, Inc.
- Propper Manufacturing Co., Inc.
- SciCan Ltd.
- STERIS plc
- Terragene S.A.
- Thermo Fisher Scientific Inc.
- Tuttnauer
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
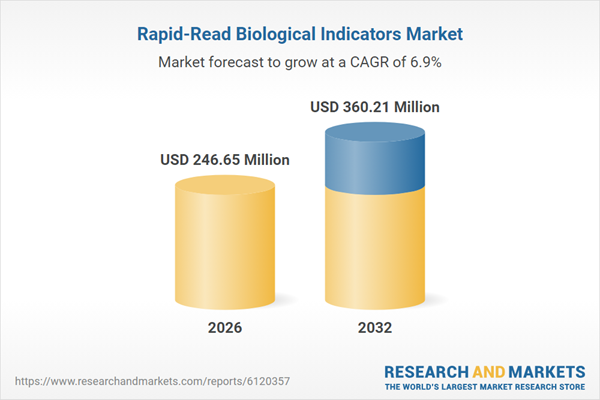
| Estimated Market Value ( USD | $ 246.65 Million |
| Forecasted Market Value ( USD | $ 360.21 Million |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |