Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative orientation to lower limb peripheral arterial device dynamics shaped by patient complexity, procedural choice, and evolving care-delivery priorities
Peripheral arterial disease of the lower limb presents a complex clinical challenge that intersects ageing demographics, comorbidity burdens, and evolving expectations for minimally invasive care. This introduction contextualizes the interventional device landscape within those dynamics and frames why stakeholders across hospitals, ambulatory surgical centers, and specialty clinics must reassess strategy and capability. Procedural innovation, shifting care settings, and new clinical evidence are converging to change how clinicians select tools and techniques for limb salvage and symptom relief.Device diversity plays a central role in this environment. Based on device type, the pathway of care includes aspiration devices available in manual and mechanical formats, atherectomy systems spanning directional, laser, orbital, and rotational modalities, balloon catheters configured as bare or drug-coated balloons, and stent systems available as bare metal or drug-eluting options. These device choices map directly to procedural decisions and to distinct skill sets required of operators and support teams. Equally important, end-user settings vary from ambulatory surgical centers to hospitals and specialty clinics, each with different throughput expectations, infrastructure, and procurement processes.
As clinicians refine indications and as manufacturers advance device design and materials, the balance between acute procedural success and mid-term durability becomes central to device selection. In the paragraphs that follow, this executive summary synthesizes transformative trends, tariff-related implications, segmentation-level insights, regional differentiation, competitive dynamics, and actionable recommendations that together define the near-term strategic agenda for leaders in lower limb artery interventional devices.
A synthesis of the major technological, clinical practice, and care‑delivery shifts that are redefining lower limb arterial intervention strategies and procurement behaviors
The landscape for interventional devices in lower limb arterial disease is undergoing transformative shifts driven by clinical demand, technological refinement, and changes in care delivery models. Therapeutic innovation has moved beyond incremental improvements to a focus on combination strategies that pair plaque-modification tools with drug-delivery platforms to address lesion complexity and restenosis risk. Meanwhile, imaging and intraprocedural guidance have become more integrated, enabling precision treatment that can reduce fluoroscopy time and improve lesion-crossing success. These clinical advances are complemented by modular procedural approaches that favor less invasive sessions and faster patient throughput.Concurrently, the site-of-care transition towards ambulatory surgical centers and specialized outpatient units is altering purchasing behavior and capital allocation. Operators increasingly value disposables and single-use systems that simplify sterilization logistics and reduce turnover time. As reimbursement models evolve and payers emphasize value-based outcomes, manufacturers must demonstrate durable clinical benefit and cost-effectiveness to secure adoption. Regulatory pathways are also shaping product rollouts; accelerated approval mechanisms for devices with strong clinical evidence coexist with heightened scrutiny on safety events, which in turn affects post-market surveillance obligations.
Finally, supply chain resilience and manufacturing flexibility are rising to the fore. The emphasis on local production, diversified supplier networks, and component traceability reflects lessons learned from recent global disruptions. Taken together, these shifts create both the impetus and the opportunity for companies to innovate around clinical performance, operational simplicity, and strategic partnerships that align with how care is now delivered.
An assessment of how United States tariff changes in 2025 reshaped supply chains, procurement priorities, and commercial strategies across device categories and care settings
The introduction of new tariff measures in the United States in 2025 has had a cumulative impact on the supply chain dynamics and commercial calculus for lower limb arterial devices. Increased duties on certain imported components and finished devices heightened input costs for manufacturers that rely on offshore production, prompting an accelerated assessment of alternative sourcing strategies. Many device producers responded by exploring regional manufacturing hubs, renegotiating supplier agreements, and increasing inventory buffers to smooth short-term volatility. These adjustments have immediate implications for procurement cycles and capital decisions at hospitals and outpatient centers.Different device categories feel the tariff effects unevenly. Complex systems that incorporate motors, precision optics, and proprietary disposables typically rely on specialized suppliers and therefore face higher exposure to import duty adjustments. Simpler, lower-cost disposable items exhibit more price sensitivity at the point of care and can drive substitution behavior where clinicians and purchase committees seek comparable alternatives. Meanwhile, products that can be localized more readily, such as balloon catheters and certain stent platform components, become focal points for reshoring initiatives because of faster time‑to‑value and reduced tariff exposure.
Beyond direct cost effects, tariffs have produced strategic ripple effects. Procurement teams are demanding greater transparency on bill-of-materials and asking for total-cost-of-ownership analyses rather than unit price alone. Payers and integrated delivery networks are intensifying scrutiny over capital investments and consumable contracts. In response, device manufacturers are accelerating partnership models, expanding contract manufacturing relationships in low-tariff jurisdictions, and offering bundled clinical and economic evidence packages to protect adoption pathways. Thus, the tariff environment is not simply a near-term pricing issue; it is reshaping commercial models and supply chain architecture with lasting consequences for how devices are developed, manufactured, and procured.
Granular segmentation insights revealing how device types, care settings, procedural categories, and anatomical applications determine clinical adoption and commercial priorities
Segmentation provides the analytical lens necessary to understand where clinical need, technological fit, and commercial opportunity intersect across device types, end users, procedural categories, and anatomical applications. Based on device type, the clinical toolkit comprises aspiration devices available in manual and mechanical formats, atherectomy systems that include directional, laser, orbital, and rotational techniques, balloon catheters provided as bare balloons and drug-coated balloons, and stent systems offered as bare metal stents and drug-eluting stents. Each device subset aligns with specific lesion morphologies and operator preferences, and each demands dedicated training pathways, inventory management, and capital planning.When considered by end user, adoption patterns differ markedly. Ambulatory surgical centers prioritize compact, easily deployable systems with rapid turnover and minimal capital overhead. Hospitals emphasize comprehensive platforms capable of handling complex, hybrid cases and provide access to multidisciplinary teams and intensive post-procedural care. Specialty clinics often focus on targeted procedural mixes and may adopt niche technologies that address a particular procedural bottleneck or lesion subset. These distinctions influence not only purchasing decisions but also service-level agreements, clinician training investments, and product support models.
Procedure-type segmentation-spanning atherectomy procedures, percutaneous transluminal angioplasty, stenting procedures, and thrombectomy procedures-highlights differences in device interoperability and adjunctive needs. Atherectomy may favor debulking technologies and specialized catheters, angioplasty centers on balloon performance and coating chemistry, stenting requires scaffolds tailored to vessel dynamics, and thrombectomy relies on aspiration mechanics and clot-engagement design. Finally, application-specific segmentation into below-the-knee intervention, femoropopliteal intervention, and infrapopliteal intervention underscores anatomical and biomechanical constraints that inform device size, flexibility, and radial strength. Together these segmentation dimensions reveal where R&D investment and commercialization effort should concentrate to achieve clinical differentiation and operational fit.
Regional differentiation and strategic considerations that reconcile regulatory cadence, reimbursement realities, and supply chain localization across global healthcare markets
Geography remains a decisive factor in device adoption, regulatory strategy, and supply chain architecture. In the Americas, clinician networks and reimbursement frameworks support broad uptake of advanced interventional tools, although payer requirements increasingly demand demonstrable clinical benefit and cost-effectiveness. The United States, in particular, shapes standards of care that influence neighboring markets, and the region’s mix of high-volume tertiary centers and expanding outpatient sites creates diverse points of entry for new technologies.The Europe, Middle East & Africa region combines heterogeneous regulatory environments, varied reimbursement landscapes, and differing hospital infrastructure, which together require adaptable commercial strategies. In Western Europe, centralized reimbursement processes and strong clinical evidence pathways favor multi-center studies and consolidated contracts, whereas in certain Middle Eastern and African markets, relationship-driven procurement and localized distribution networks play a more prominent role. Manufacturers that tailor regulatory filings and value propositions to these nuances can gain earlier traction.
Asia-Pacific demonstrates rapid adoption of minimally invasive techniques in urban centers alongside an expanding base of regional manufacturing capacity. Regulatory harmonization efforts and active investment in local production are enabling faster product registrations and cost-competitive sourcing. Across all regions, the interplay between local manufacturing decisions, tariff exposures, and clinical trials infrastructure informs how companies prioritize market entry and scale-up. In sum, regional strategy must account for regulatory cadence, reimbursement imperatives, clinical ecosystem maturity, and supply chain considerations to succeed internationally.
Competitive and collaborative dynamics among established medtech firms, specialized innovators, and contract manufacturers shaping portfolio strategies and market access
Competitive dynamics in the lower limb artery interventional device space reflect a mix of established medical device firms, specialized innovators, contract manufacturers, and clinical research collaborators. Incumbent players maintain advantages in distribution scale, clinician relationships, and infrastructure for post-market surveillance, while emerging entrants often focus on narrow clinical problems with differentiated engineering or drug‑delivery approaches. These dynamics create a fertile environment for partnerships that combine clinical expertise with manufacturing efficiency and for transactions that accelerate access to complementary technologies.Product portfolio breadth influences negotiating leverage with large hospital systems and group purchasing organizations, while niche devices can command higher per-procedure margins if clinical evidence supports superior outcomes. Simultaneously, service elements such as training programs, clinical support during early adoption, and data‑driven outcome tracking have become critical differentiators. Intellectual property around coating chemistries, atherectomy mechanics, and stent architecture continues to shape competitive moats, but it also motivates cross-licensing and joint development agreements that can speed innovation.
Finally, the ability to deliver integrated clinical and economic evidence-real-world registries, head-to-head studies, and health economic models-determines the pace and scale of adoption. Companies that align product development with payer and provider decision criteria will be better positioned to secure long-term contracts and clinician preference in an increasingly evidence-driven market.
Practical strategic moves for manufacturers and providers to secure clinical adoption, fortify supply chains, and demonstrate payer‑relevant value across diverse care settings
Industry leaders should pursue a coordinated set of actions to capture clinical adoption, mitigate supply risk, and demonstrate economic value. First, prioritize clinical evidence generation that addresses durability and reintervention endpoints relevant to payers and high-volume operators, and align study designs with common procedural categories and anatomical applications to maximize generalizability. Simultaneously, invest in educational programs that accelerate operator proficiency across atherectomy techniques, balloon‑first strategies, and stent deployment nuances, thereby reducing variability in outcomes and building clinician advocacy.Second, reassess manufacturing and sourcing strategies in light of tariff and supply-chain volatility. Where feasible, qualify regional manufacturing partners, diversify component suppliers, and introduce contractual clauses that share currency and duty risk with channel partners. These steps will improve resiliency and provide commercial flexibility when negotiating with hospital systems and ambulatory sites. Third, refine commercial models to match end-user needs: offer capital-light options and procedural bundles for ambulatory surgical centers while providing comprehensive platform support and hybrid-procedure capabilities for hospital networks.
Fourth, develop integrated value propositions that pair clinical outcomes data with total-cost-of-care analyses, facilitating payer dialogue and pathway inclusion. Fifth, pursue strategic partnerships with imaging and digital-health providers to enhance intraprocedural guidance and post-procedure follow-up, thereby strengthening the product narrative. Finally, maintain regulatory and reimbursement intelligence to anticipate regional differences and accelerate market entry through adaptive trial designs and targeted evidence packages. By implementing these measures, leaders can reconcile short-term operational pressures with long-term clinical adoption objectives.
A concise overview of the multi‑method research approach combining clinical literature, expert interviews, registry analysis, and supply‑chain review to ensure robust findings
This analysis synthesizes primary and secondary research methodologies to triangulate clinical, commercial, and operational insights. We reviewed peer-reviewed clinical literature, procedural registries, and publicly available regulatory documentation to establish a foundation of evidence on safety and efficacy across device classes. Additionally, we conducted structured interviews with interventional clinicians, procurement leaders, biomedical engineers, and health-economics specialists to capture real-world practice patterns and purchasing priorities.Complementing qualitative inputs, the methodology incorporated a review of product portfolios, patent landscapes, and contract manufacturing footprints to assess supply-chain risk and localization potential. We also examined coding and reimbursement frameworks across major geographies to align clinical outcomes with payer decision criteria. Throughout, findings were validated through cross-referencing multiple sources and by subject-matter expert review to reduce bias and improve reliability.
Limitations of the approach include variability in publicly available outcome reporting across device types and rapidly evolving tariff and regulatory environments that may change after data collection. To mitigate these constraints, the research emphasizes scenario-based analysis and recommends ongoing evidence generation and surveillance to inform adaptive commercial strategies.
A decisive conclusion linking clinical innovation, operational resilience, and payer alignment as the prerequisites for sustainable adoption and improved patient outcomes
The lower limb artery interventional device landscape is at an inflection point characterized by converging clinical needs, technological maturation, and shifting economic incentives. Opportunities exist across device categories and anatomical applications for solutions that demonstrably improve durability while simplifying procedural workflows. However, realizing these opportunities requires coordinated action across evidence generation, manufacturing strategy, and targeted commercialization to meet the distinct needs of hospitals, ambulatory surgical centers, and specialty clinics.As tariffs and supply-chain pressures reshape cost structures, manufacturers and providers must adopt flexible sourcing and pricing approaches while continuing to invest in clinical data that supports long-term value. Ultimately, success will accrue to organizations that integrate clinical excellence with operational resilience and that engage payers and providers early to align incentives. This conclusion underscores a strategic imperative: innovation must be linked to measurable outcomes and pragmatic delivery models to achieve sustainable adoption and to improve patient care in the years ahead.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Lower Limb Artery Interventional Devices Market
Companies Mentioned
The key companies profiled in this Lower Limb Artery Interventional Devices market report include:- Abbott Laboratories
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Cook Medical LLC
- Johnson & Johnson
- Koninklijke Philips N.V.
- Medtronic plc
- MicroPort Scientific Corporation
- Terumo Corporation
- W. L. Gore & Associates, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
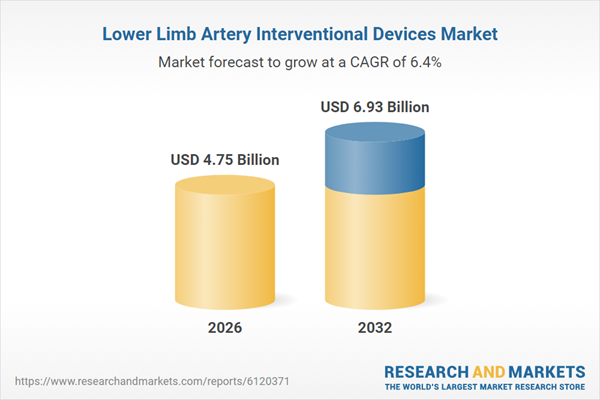
| Estimated Market Value ( USD | $ 4.75 Billion |
| Forecasted Market Value ( USD | $ 6.93 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |