Speak directly to the analyst to clarify any post sales queries you may have.
Intervertebral disc endoscopy is redefining minimally invasive spine care as technology, economics, and patient expectations converge
Intervertebral disc endoscopy has moved from a niche option to a strategic capability in contemporary spine care, driven by the clinical push toward smaller incisions, faster recovery, and more predictable perioperative pathways. As hospitals and ambulatory surgical centers reassess how to deliver high-quality outcomes under increasing cost and capacity constraints, endoscopic approaches are being evaluated not only as a surgical technique, but also as an operational lever that can improve throughput, reduce length of stay, and support patient satisfaction goals.At the same time, the technology stack behind disc endoscopy is advancing quickly. High-definition visualization, refined working-channel endoscopes, and specialized instruments are broadening the addressable case mix, especially for carefully selected lumbar pathologies. This has encouraged more surgeons to consider endoscopy as an extension of minimally invasive spine surgery rather than an entirely separate discipline.
However, adoption is not automatic. Stakeholders must navigate surgeon learning curves, capital allocation for towers and instrumentation, and variable payer and guideline environments. Consequently, decision-makers increasingly demand evidence of real-world outcomes, standardized training pathways, and total cost-of-care narratives that can withstand scrutiny from value analysis committees.
Against this backdrop, the competitive landscape is intensifying as established spine companies defend share and specialized endoscopy firms expand indications, instrument breadth, and training ecosystems. The executive challenge is to prioritize investments that align with clinical demand, procurement realities, and supply chain resilience while maintaining a credible, patient-centric story.
Platform consolidation, outpatient migration, and data-driven value arguments are transforming how disc endoscopy is adopted and scaled
The landscape is undergoing a pronounced shift from technique-led adoption to platform-led strategy. Early market development often centered on individual surgeon champions and a limited set of procedures. Now, the center of gravity is moving toward integrated endoscopic ecosystems that combine visualization, access, resection tools, irrigation control, and imaging compatibility. This shift favors suppliers that can offer coherent procedural workflows, predictable consumable availability, and standardized training modules.In parallel, the site of care is evolving. As procedural efficiency improves and anesthesia protocols mature, more disc endoscopy cases are being considered for outpatient settings where appropriate, especially when patient selection and postoperative support are robust. This change increases the importance of service models that minimize downtime, simplify reprocessing, and ensure rapid instrument turnaround.
Another transformative shift is the rising role of data, not only in clinical outcomes but also in operational metrics. Hospitals and surgical centers increasingly monitor case times, readmissions, and patient-reported outcomes to justify adoption and to support contracting decisions. Vendors are responding by packaging education with outcome tracking tools, cadaver labs, and proctoring networks that help shorten the time to surgeon proficiency.
Finally, the competitive basis is expanding beyond optics and instruments into training access, procedural breadth, and compatibility with adjacent technologies such as navigation or robotics in selected workflows. As these elements converge, executive teams must treat intervertebral disc endoscopy as a strategic portfolio domain that touches capital planning, physician alignment, and pathway standardization rather than a standalone product purchase.
United States tariff changes in 2025 are reshaping sourcing, pricing negotiations, and product design choices across disc endoscopy supply chains
United States tariff dynamics in 2025 are amplifying supply chain and pricing complexity for intervertebral disc endoscopy, particularly where components or finished goods cross borders multiple times before reaching sterile processing and the operating room. Endoscopy systems often rely on precision optics, camera modules, light sources, and specialty alloys for instruments-inputs that are frequently sourced through global networks. When tariff exposure increases or becomes less predictable, procurement teams tend to demand clearer cost breakdowns and more stable pricing commitments.As a result, manufacturers and distributors are increasingly prioritizing supply chain re-engineering. This includes shifting certain assembly steps closer to end markets, dual-sourcing critical components, and redesigning instruments to reduce dependency on tariff-sensitive materials where feasible. Even when these steps improve resilience, they can introduce transitional friction, such as validation timelines, regulatory documentation updates, and temporary inventory buffering.
Tariffs also influence contracting behavior. Providers under budget pressure may delay capital purchases or seek flexible financing and bundled pricing that spreads cost over time. This can favor vendors with strong balance sheets, robust field service networks, and the ability to offer comprehensive procedure packs that simplify purchasing. Conversely, smaller innovators may face margin compression if they cannot pass incremental costs through to customers.
Importantly, tariff-related uncertainty can accelerate interest in local sterilization compatibility and durable, reusable instrument strategies where clinically appropriate, as facilities re-evaluate the economics of disposable versus reusable components. For executive leaders, the practical takeaway is that 2025 tariff conditions are not merely a finance issue; they shape product design, channel strategy, inventory policy, and the narrative used to defend adoption in value analysis settings.
Segmentation insights show diverging adoption triggers across products, procedures, care settings, and purchasing pathways in disc endoscopy
Segmentation reveals that decision drivers vary substantially depending on how the market is viewed through product, application, end user, and purchasing behavior lenses. When the focus shifts from endoscopy systems and visualization towers to specialized working-channel endoscopes, access kits, and powered instruments, the value proposition becomes less about the initial capital outlay and more about procedural reliability, ergonomics, and the consistency of consumable supply. In this context, purchasing teams often evaluate not only per-case spend but also reprocessing burden, instrument longevity, and the vendor’s ability to maintain readiness through preventive maintenance and rapid replacement.From an application standpoint, lumbar disc herniation remains a core clinical anchor, yet segmentation highlights increasing differentiation in how providers assess endoscopic decompression for stenosis-related complaints and recurrent cases. This is where training depth and evidence clarity become decisive, because the clinical pathway must be credible to both surgeons and payers. As case complexity increases, facilities tend to favor broader instrument portfolios and stronger intraoperative visualization options that can support variation in anatomy and pathology without frequent tool changes.
End-user segmentation underscores the growing importance of ambulatory surgical centers alongside hospitals, but the drivers differ. Hospitals may emphasize multidisciplinary spine programs, revision readiness, and alignment with imaging and sterile processing standards. Ambulatory surgical centers often prioritize turnover time, simplified tray logistics, and predictable supply agreements that support high-volume scheduling. Consequently, the same endoscopic platform may be positioned differently depending on whether the customer values complex-case flexibility or streamlined outpatient execution.
Finally, segmentation by purchasing pathway-capital equipment committees, service contracts, distributor-led procurement, or surgeon preference-driven adoption-clarifies why go-to-market strategies must be customized. Where committees dominate, vendors must present total cost-of-ownership logic, training plans, and compliance documentation. Where surgeon preference is influential, hands-on demos, peer-to-peer education, and procedural support may be the pivotal levers. Treating these segmentation dimensions as interconnected rather than isolated helps explain why adoption can accelerate in one customer cohort while remaining slow in another.
Regional insights highlight how reimbursement, training infrastructure, and outpatient readiness shape disc endoscopy adoption across major geographies
Regional dynamics indicate that adoption and commercialization pathways are strongly shaped by reimbursement structure, surgeon training density, and the maturity of outpatient spine ecosystems. In the Americas, demand is closely tied to operational efficiency goals and the migration of appropriate cases toward outpatient settings, which elevates the importance of service reliability and standardized training support. Decision-making often runs through value analysis processes that require outcome narratives alongside procurement discipline.In Europe, the market environment is shaped by country-level health system structures, procurement centralization in some settings, and a strong emphasis on clinical evidence and guideline alignment. Adoption often advances through centers of excellence and academic influence, which can create durable reference sites but may extend the time required to move from pilot programs to broad rollout. In addition, cross-border variability in coding and reimbursement can lead companies to pursue highly tailored commercialization strategies rather than uniform regional playbooks.
The Middle East & Africa presents a different profile, where leading tertiary centers can adopt advanced endoscopic spine capabilities rapidly, especially when aligned with national healthcare modernization programs. Nevertheless, scale-up can be uneven due to differences in infrastructure, access to skilled personnel, and distributor capabilities. Success often depends on training partnerships, reliable service coverage, and instrument availability that can withstand longer replenishment cycles.
In Asia-Pacific, growth is influenced by large patient populations, expanding hospital capacity, and increasing interest in minimally invasive techniques. The region also features strong manufacturing capabilities in select markets and a growing ecosystem of surgeon training programs. However, heterogeneity remains significant, with adoption varying by regulatory pathways, pricing sensitivity, and the relative availability of outpatient surgery infrastructure. Across all regions, companies that combine clinical education with adaptable commercial models are best positioned to navigate local constraints while maintaining global consistency in quality and outcomes.
Company dynamics are shifting from standalone endoscopic tools to full procedural ecosystems backed by training, service depth, and evidence credibility
The competitive environment in intervertebral disc endoscopy spans diversified spine and surgical technology leaders as well as specialized endoscopy-focused innovators. Larger companies typically compete through breadth-offering complementary implants, access systems, imaging integration, and established hospital contracting relationships. Their advantages often include scalable field service, structured education programs, and the ability to bundle offerings across multiple spine procedures, which can be persuasive for integrated delivery networks and high-volume centers.Specialist players, by contrast, frequently compete through depth in endoscopic workflows, with instrument sets optimized for specific approaches and a strong emphasis on surgeon training communities. They may lead with user-centric design, procedural efficiency, and rapid iteration of tools that respond to surgeon feedback. In many cases, these companies also invest heavily in proctoring networks and hands-on training that accelerates adoption among early and mid-career surgeons.
Across both groups, differentiation increasingly hinges on three factors: the clarity of the learning pathway, the robustness of the procedural ecosystem, and the credibility of clinical and economic evidence. Visualization quality and instrument precision remain table stakes, but customers are placing more weight on tray rationalization, reprocessing compatibility, uptime guarantees, and the availability of local technical support.
Partnerships and co-development are also becoming more visible, especially where camera systems, light sources, and imaging interfaces require specialized expertise. As consolidation and collaboration continue, executive teams should monitor which companies are building end-to-end procedural platforms versus those focusing narrowly on components, because this strategic choice influences pricing power, switching costs, and long-term customer retention.
Actionable recommendations emphasize training scalability, operational value, tariff-resilient sourcing, and evidence generation beyond clinical endpoints
Industry leaders should treat training as a core commercial asset rather than a supporting activity. Investing in standardized curricula, regional cadaver labs, and structured proctorship can materially reduce adoption friction and protect customer satisfaction during the learning curve. Just as important, aligning training with measurable competency milestones helps reassure administrators that patient safety and efficiency will improve predictably over time.A second priority is to redesign offerings around operational outcomes. Facilities want fewer trays, faster turnover, and reliable availability of high-wear components. Vendors can respond by rationalizing instrument sets, strengthening preventive maintenance programs, and offering service-level commitments that reduce downtime risk. When paired with transparent reprocessing guidance and validated compatibility with common sterilization workflows, these steps help elevate endoscopy from a surgeon preference purchase to an institutionally endorsed capability.
Leaders should also harden their supply chains against tariff and logistics volatility. Dual-sourcing critical components, regionalizing final assembly where practical, and improving demand planning for consumables can reduce sudden disruptions. In parallel, pricing strategy should anticipate procurement scrutiny; value-based bundles and flexible financing can be more persuasive than unit discounts when customers are balancing capital constraints with clinical goals.
Finally, companies should expand evidence generation beyond traditional endpoints. Patient-reported outcomes, time-to-return-to-work proxies, and operational measures such as case duration and readmission patterns resonate with both clinicians and administrators. Embedding data collection tools into training and onboarding can make evidence generation an ongoing capability, strengthening competitive positioning and supporting payer and committee discussions.
Methodology combines stakeholder interviews, workflow analysis, and policy review to produce decision-ready insights for disc endoscopy strategy
The research methodology integrates multiple stages designed to capture technology evolution, clinical practice realities, and commercialization constraints in intervertebral disc endoscopy. The work begins with comprehensive secondary review of regulatory pathways, device classifications, clinical guidance trends, and publicly available company materials to establish a baseline understanding of platforms, procedural approaches, and competitive positioning.This foundation is complemented by structured primary engagement with stakeholders across the value chain, including clinicians, administrators, procurement professionals, distributors, and industry participants. Interviews are designed to surface decision criteria, adoption barriers, training requirements, service expectations, and the practical implications of supply chain variability. Insights are triangulated across roles to reduce single-perspective bias and to distinguish stated preferences from operational constraints.
Analytical framing then organizes findings around product and workflow architecture, site-of-care dynamics, and region-specific commercialization pathways. Special attention is paid to how tariff conditions, sterilization practices, and contracting models influence adoption. Where viewpoints diverge, the methodology emphasizes reconciliation through follow-up validation and cross-checking against documented purchasing and clinical workflow norms.
Finally, the output is synthesized into decision-ready insights that prioritize what executives can act on: where adoption is most sensitive to training and service depth, which customer segments value bundled ecosystems, and how regional differences alter the path to scale. The methodology is designed to remain current by incorporating recent policy developments and by reflecting ongoing shifts in outpatient spine care delivery.
Conclusion ties together maturity, resilience, and execution excellence as the decisive factors shaping disc endoscopy’s next chapter
Intervertebral disc endoscopy is entering a phase where the winners will be determined less by novelty and more by execution. Technology improvements have expanded the feasible procedure set, but customers now evaluate platforms through the lens of reproducible outcomes, training accessibility, and operational fit within busy surgical environments. The market’s maturation is pushing stakeholders toward standardized pathways that can be adopted reliably across surgeons and sites of care.Tariff and supply chain pressures in 2025 further reinforce the need for resilience. Companies that proactively adapt sourcing, maintain consistent service levels, and communicate pricing logic transparently will be better positioned to navigate procurement scrutiny. Meanwhile, providers will continue to demand credible evidence that supports both clinical and economic decision-making, particularly as outpatient migration intensifies.
Segmentation and regional perspectives make clear that there is no single adoption playbook. Product architecture, procedure focus, end-user setting, and purchasing pathway all shape what customers value, while regional reimbursement and training ecosystems determine how quickly programs can scale. Organizations that align these dimensions into coherent strategy will be best equipped to translate endoscopic capability into sustainable growth.
Ultimately, disc endoscopy’s trajectory will be defined by integrated ecosystems-tools, training, service, and data-delivered with discipline. Executive teams that act now to strengthen these pillars can capture momentum while building long-term trust with clinicians and healthcare systems.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
19. China Intervertebral Disc Endoscopy Market
Companies Mentioned
The key companies profiled in this Intervertebral Disc Endoscopy market report include:- Alphatec Spine, Inc.
- Arthrex, Inc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Conmed Corporation
- Globus Medical, Inc.
- Integra LifeSciences Corporation
- Joimax GmbH
- Karl Storz SE & Co. KG
- Maxcess International
- Medacta International SA
- Medtronic plc
- NuVasive, Inc.
- Olympus Corporation
- Precision Spine, Inc.
- Richard Wolf GmbH
- Smith & Nephew plc
- Spinal Elements, Inc.
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
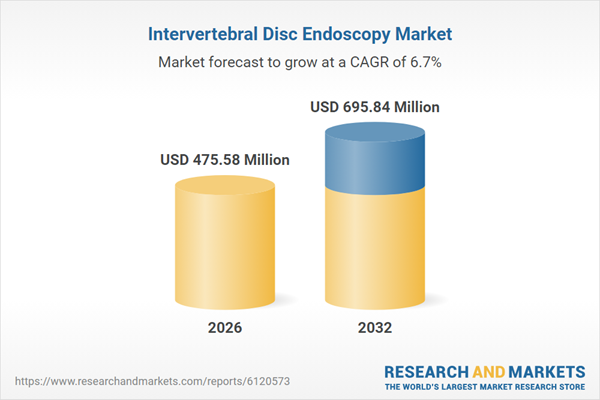
| Estimated Market Value ( USD | $ 475.58 Million |
| Forecasted Market Value ( USD | $ 695.84 Million |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |