Speak directly to the analyst to clarify any post sales queries you may have.
Strategic importance of xanthan gum in pharmaceuticals is rising as rheology control, compliance rigor, and patient-centric formulation priorities converge
Xanthan gum has moved far beyond its legacy role as a broadly useful thickener, becoming a deliberately engineered excipient choice in pharmaceutical development where rheology, stability, and patient experience are tightly interdependent. In many liquid and semi-solid dosage forms, it contributes to controlled viscosity, improved suspension stability, and consistent dose delivery, while also supporting texture and mouthfeel expectations that can influence adherence. As a result, it increasingly sits at the intersection of formulation science, quality assurance, and supply chain governance rather than being treated as a simple commodity hydrocolloid.This market landscape is defined by the need to balance performance and compliance across diverse product formats. Developers seek predictable pseudoplastic behavior for ease of pouring, spreading, and syringeability, but they also need compatibility with actives, buffers, electrolytes, and co-excipients. At the same time, quality teams demand rigorous microbiological control, traceability, and change management, while procurement teams confront supplier consolidation, logistics volatility, and shifting trade policies. These pressures have elevated the importance of grade selection, specification discipline, and supplier qualification strategies.
Against this backdrop, the pharmaceutical use of xanthan gum is increasingly shaped by two parallel trends: the pursuit of patient-centric design and the tightening of excipient governance. Patient-centric design pushes formulators toward excipients that can enable palatable, stable, easy-to-administer products without adding unnecessary complexity. Excipient governance emphasizes risk-based oversight, including vulnerability to contamination, variability, and supply disruption. Together, these trends make xanthan gum a strategic input whose selection and management can influence development timelines, regulatory confidence, and product lifecycle resilience.
Quality-by-design expectations, tighter excipient oversight, and patient-centric dosage form innovation are redefining how xanthan gum competes and qualifies
The landscape for pharmaceutical xanthan gum is being reshaped by a more demanding definition of “fit for purpose.” Historically, many teams viewed xanthan gum primarily through viscosity targets and basic compendial alignment. Today, leading developers evaluate it through a multi-dimensional lens that includes particle characteristics, hydration behavior, ionic tolerance, bioburden risk, and lot-to-lot reproducibility under real formulation conditions. This shift reflects a broader movement toward quality-by-design and stronger linking of excipient attributes to critical quality attributes in the finished product.At the same time, the industry is adapting to heightened scrutiny of excipient supply chains. More companies are formalizing supplier quality agreements, tightening change notification expectations, and increasing audit depth, especially for fermentation-derived materials. This has elevated the value of transparent manufacturing controls, robust environmental monitoring, and clear traceability from raw materials through downstream processing and packaging. In parallel, sustainability expectations are becoming more concrete, with attention on fermentation inputs, energy usage, wastewater treatment, and responsible sourcing narratives that can withstand customer and regulator questions.
Formulation innovation is also driving change in where and how xanthan gum is deployed. Patient preferences and adherence considerations are encouraging more sophisticated oral liquids and reconstitutable formats, while topical and mucosal products continue to demand stable, elegant rheology across temperature swings and repeated use. Additionally, as developers pursue simpler labels and reduce reliance on certain synthetic rheology modifiers, xanthan gum’s “naturally derived” positioning can become an advantage, provided that quality, microbial control, and allergen-related concerns are addressed proactively.
Finally, the competitive environment is shifting toward service-oriented differentiation. Suppliers and distributors are increasingly expected to support application troubleshooting, documentation readiness, and rapid responsiveness to deviations or changes. As a result, technical support capabilities, documentation completeness, and demonstrated regulatory readiness are becoming as influential as base material performance, especially for customers working under accelerated development timelines or strict internal governance.
United States tariffs in 2025 may reshape landed costs, supplier qualification urgency, and contract structures across pharmaceutical xanthan gum supply chains
The introduction of United States tariffs in 2025 has the potential to ripple through the pharmaceutical xanthan gum value chain in ways that extend beyond direct price effects. When tariffs touch upstream inputs, fermentation intermediates, or finished excipient imports, they can alter landed-cost calculations and lead companies to revisit sourcing footprints. Even when a specific xanthan gum grade is not explicitly targeted, the broader tariff environment can influence freight patterns, customs processing times, and supplier allocation priorities, all of which matter to manufacturers seeking uninterrupted supply for regulated products.One immediate impact area is procurement strategy. Buyers may accelerate qualification of alternate suppliers, add dual-sourcing requirements, or increase safety stock to buffer border-related variability. However, pharmaceuticals cannot switch excipients as freely as other industries, so the true cost is often embedded in quality work: change control, comparability assessment, internal validation activities, and documentation updates. Consequently, tariffs can indirectly increase operational burden by forcing earlier and more frequent risk evaluations and by elevating the value of suppliers that can provide stable documentation, consistent specifications, and predictable lead times.
Tariffs can also reshape negotiating dynamics between manufacturers, distributors, and end users. Contracts may shift toward clearer incoterms, defined responsibility for tariff exposure, and stronger commitments on allocation during supply tightness. In parallel, some firms may explore local or regional processing and packaging as a mitigation tactic, aiming to reduce exposure tied to import classification or to streamline customs complexity. This can create new partnership models where bulk material is sourced globally while final conversion steps occur closer to the point of use.
From a regulatory and quality perspective, the tariff environment reinforces the need for disciplined supplier governance. Companies that treat excipient management as a strategic capability-maintaining updated technical packages, robust vendor scorecards, and proactive change notification channels-will be better positioned to absorb policy-driven disruptions. Over time, the cumulative impact of tariffs may not simply be a reallocation of suppliers; it may be a structural shift toward more resilient qualification practices and a greater preference for suppliers with diversified manufacturing footprints and proven compliance maturity.
Segmentation shows pharmaceutical xanthan gum demand varies sharply by grade assurance, dosage form rheology needs, end-user governance, and channel expectations
Segmentation patterns reveal that pharmaceutical demand for xanthan gum is best understood as a set of use cases with distinct performance and compliance thresholds rather than a single uniform requirement. When the market is viewed by product type, the core distinction is the degree of purification and documentation readiness, which influences suitability for regulated drug products versus less stringent healthcare applications. Higher-assurance grades tend to be pulled into programs where microbial limits, traceability, and tight rheological reproducibility are non-negotiable, while other grades can serve roles where the risk profile and regulatory burden are comparatively lighter.When analyzed by grade and functionality, viscosity targets alone do not explain purchasing decisions. Buyers frequently prioritize hydration behavior, stability under shear, and tolerance to salts and pH ranges that mirror real formulations. This is particularly relevant where xanthan gum supports suspension of insoluble actives or contributes to controlled flow in multi-dose containers. In practice, formulators often evaluate xanthan gum alongside other hydrocolloids or cellulosics, and the selection hinges on how it performs in the full excipient system, how robustly it withstands processing, and how consistent it remains across lots.
Considering dosage form segmentation, oral liquids and reconstitutable products emphasize pourability, dose uniformity, and palatability, pushing the need for predictable pseudoplasticity and stable viscosity over shelf life. Topical and semi-solid systems, including gels and creams, focus on spreadability, residue profile, and sensory elegance while maintaining stability across temperature excursions and repeated opening. In ophthalmic and other sensitive mucosal applications, the tolerance for variability narrows further, and stakeholders scrutinize particulate control, endotoxin risk, and documentation depth more intensely.
Segmentation by end user highlights different buying behaviors. Large pharmaceutical manufacturers often demand comprehensive quality agreements, extensive audits, and established change control practices, valuing long-term reliability over opportunistic pricing. Contract development and manufacturing organizations tend to require agility, multi-client documentation readiness, and rapid technical responses because they must satisfy diverse sponsor requirements under compressed timelines. Meanwhile, compounding and smaller specialty manufacturers may focus on availability, manageable minimum order quantities, and practical guidance, though they still face growing expectations for traceability and consistent quality.
Finally, segmentation by distribution channel shapes how value is delivered. Direct supplier relationships can offer stronger technical collaboration and clearer change notification pathways, which are critical for regulated supply continuity. Authorized distributors can add resilience through inventory positioning, local warehousing, and streamlined logistics, especially when global trade conditions are volatile. Across these segmentation lenses, the market’s defining theme is that “pharmaceutical xanthan gum” is increasingly a specification-driven, application-dependent decision where documentation, risk management, and technical support weigh as heavily as rheology.
Regional demand patterns reflect different regulatory strictness, manufacturing concentration, and logistics resilience across the Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional dynamics for pharmaceutical xanthan gum reflect how regulation, manufacturing concentration, and supply chain infrastructure interact. In the Americas, demand is strongly influenced by mature regulatory expectations and the operational reality that excipient changes can trigger significant internal qualification work. Buyers tend to prefer suppliers with consistent documentation and established quality practices, while also seeking logistics reliability given the sensitivity of liquid and semi-solid product supply. In addition, trade policy uncertainty encourages a more explicit focus on dual sourcing, inventory planning, and contract terms that clearly allocate risk.In Europe, the emphasis on product quality systems and lifecycle management drives careful excipient oversight, including expectations around traceability, change control, and alignment with compendial standards. Sustainability considerations also play a more visible role in procurement discussions, prompting deeper questions about fermentation inputs, waste management, and supplier transparency. These factors support demand for suppliers that can demonstrate not only technical fitness but also mature governance and responsiveness during audits and post-approval change events.
The Middle East and Africa present a different mix of drivers, where access, import logistics, and distributor networks can be decisive. Pharmaceutical manufacturers and healthcare producers in the region often value dependable availability and documentation that supports regulatory submissions, while navigating varying national requirements and lead-time constraints. As local manufacturing capabilities expand in select markets, there is growing interest in stable regional supply arrangements and in partners that can provide on-the-ground technical and quality coordination.
In Asia-Pacific, the landscape is shaped by a combination of expanding pharmaceutical production capacity and strong participation in global supply chains. Buyers range from globally regulated exporters to domestic-focused manufacturers, creating a wide spectrum of documentation needs and price-performance expectations. Regional supply strength can support shorter lead times, but the market also places high importance on consistent quality, robust microbiological control, and clear traceability for products destined for stringent regulatory jurisdictions. Across all regions, the common thread is an increasing preference for suppliers that can combine dependable logistics with auditable quality systems and application-aware technical support.
Competition increasingly rewards suppliers with auditable quality culture, strong technical service, resilient manufacturing footprints, and disciplined change management for regulated use
The competitive environment for pharmaceutical xanthan gum is characterized by a blend of large-scale hydrocolloid producers, fermentation specialists, and distribution partners that package and position compliant grades for regulated applications. Leading companies differentiate through consistent manufacturing controls, tight specifications, and the ability to support customer audits with complete documentation packages. Increasingly, customers expect suppliers to demonstrate disciplined change management and to provide timely, transparent communication when raw material inputs, sites, or processes evolve.A key point of differentiation is technical service depth. Suppliers that can actively support formulation troubleshooting-such as optimizing hydration, managing electrolyte sensitivity, or preventing viscosity drift over time-tend to become preferred partners, especially for oral liquid suspensions and topical systems. This service component is often paired with application data, sample support, and guidance on processing order, shear exposure, and compatibility with preservatives or co-solvents. In regulated settings, the ability to translate this support into clear, controlled documentation can accelerate internal approvals and reduce development friction.
Another competitive axis is supply resilience. Companies with diversified manufacturing footprints, robust inventory strategies, and reliable logistics partners can reduce the operational risk faced by drug manufacturers. This matters because shortages can force painful reformulation or production interruptions, and even short disruptions can trigger cascading impacts across product portfolios. As a result, buyers increasingly evaluate suppliers not only on current performance, but also on demonstrated continuity planning, allocation practices, and responsiveness during disruptions.
Finally, credibility in pharmaceutical contexts depends on quality culture. Firms that invest in microbiological control, traceability, complaint handling, and preventive action systems earn greater trust during supplier qualification. Over time, this reinforces a market structure where long-term relationships are built on auditable consistency and problem-solving capability, rather than on transactional pricing alone.
Leaders can win by elevating excipient governance, hardening supplier continuity plans, standardizing processing know-how, and strengthening lifecycle documentation discipline
Industry leaders can strengthen their position by treating xanthan gum as a critical excipient input rather than a routine thickener. The first priority is to link material attributes to product performance using a risk-based framework that connects rheology, microbiological limits, and stability behavior to critical quality attributes of the finished dosage form. This approach supports clearer specifications, faster deviation triage, and better alignment between R&D and Quality when scale-up introduces shear, temperature, or mixing-order variability.Next, leaders should upgrade supplier governance with a focus on continuity and transparency. Dual sourcing strategies are valuable, but only when they are paired with realistic qualification timelines, pre-defined comparability expectations, and clear triggers for change evaluation. Quality agreements should explicitly address notification windows, raw material traceability, microbial control practices, and documentation updates. In parallel, procurement teams can reduce tariff and logistics exposure by negotiating contract terms that clarify landed-cost responsibilities and by considering regional warehousing or local conversion steps where feasible.
Formulation teams can capture additional value by standardizing best practices for hydration and processing. Xanthan gum performance is sensitive to dispersion technique, order of addition, and interactions with salts and preservatives. Establishing platform formulations or processing playbooks for common dosage forms-oral suspensions, syrups, gels, and creams-can shorten development cycles and improve batch-to-batch robustness. Moreover, stability protocols should explicitly test real-world use patterns, such as repeated opening, temperature cycling, and dispensing stress, to ensure viscosity and suspendability remain acceptable.
Finally, leaders should invest in documentation readiness across the product lifecycle. This includes maintaining current technical packages, mapping excipient changes to regulatory impact assessments, and ensuring internal stakeholders can rapidly access approved specifications and supplier statements. With tariffs and supply volatility adding uncertainty, organizations that operationalize excipient intelligence-through cross-functional reviews and structured vendor scorecards-will be better positioned to maintain continuity and protect patient supply.
Methodology combines stakeholder interviews, documentation review, compendial and regulatory referencing, and triangulation to validate pharmaceutical xanthan gum insights
The research methodology integrates primary and secondary inputs to build a practical view of how xanthan gum is specified, qualified, and adopted for pharmaceutical applications. The process begins with structured landscape mapping of the value chain, covering fermentation-based manufacturing, downstream processing, packaging and distribution models, and the quality and regulatory documentation that supports regulated use. This establishes how materials move from production to end-user qualification and identifies common points of friction.Primary research incorporates interviews and consultations with stakeholders across the ecosystem, including formulation scientists, quality and regulatory professionals, procurement leaders, and supplier technical teams. These discussions focus on real-world decision criteria such as specification tightness, microbial control expectations, audit priorities, and the operational challenges of switching or qualifying alternate sources. Feedback is then normalized into comparable themes to reduce individual bias and to highlight repeatable patterns across dosage forms and regions.
Secondary research draws from publicly available compendial references, regulatory guidance, trade and customs documentation frameworks, and technical literature on hydrocolloid functionality in pharmaceutical systems. Company materials such as technical data sheets, safety documentation, quality statements, and product catalogs are reviewed to understand how suppliers position grades and how documentation packages align with buyer expectations. Where appropriate, information is cross-checked across multiple independent sources to improve reliability.
Finally, the analysis uses triangulation to reconcile differences between what stakeholders report, what documentation indicates, and what application science suggests. Insights are stress-tested for internal consistency, ensuring that conclusions reflect plausible cause-and-effect relationships rather than assumptions. The result is an evidence-based narrative designed to support decision-making in formulation, quality assurance, and sourcing without relying on speculative sizing or unsupported claims.
Xanthan gum is shifting from commodity excipient to risk-managed performance enabler as compliance expectations and supply uncertainty intensify across pharma
Xanthan gum’s role in pharmaceuticals is becoming more strategic as the industry places greater weight on patient experience, dosage form robustness, and excipient governance. What appears to be a straightforward viscosity modifier can materially influence stability, dose uniformity, and usability, particularly in oral liquids and topical systems where rheology defines performance at the point of administration. Consequently, decisions about grade selection and supplier choice increasingly resemble broader risk management choices rather than routine purchasing.The landscape is also being reshaped by heightened supply chain scrutiny and policy-driven uncertainty. The cumulative effect of tighter oversight, sustainability expectations, and tariff-related disruptions is pushing organizations toward stronger qualification discipline, clearer documentation requirements, and more resilient sourcing models. Companies that anticipate these pressures will avoid reactive change control and reduce the probability of avoidable disruptions.
Ultimately, competitive advantage will favor organizations that connect excipient science to operational execution. By aligning formulation requirements with auditable supplier controls and pragmatic continuity planning, pharmaceutical manufacturers and partners can protect product quality, accelerate development pathways, and maintain dependable supply for patients.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Xanthan Gum for Pharmaceutical Market
Companies Mentioned
The key companies profiled in this Xanthan Gum for Pharmaceutical market report include:- A. B. Enterprises
- Antares Chem Private Limited
- Archer Daniels Midland Company
- C.E. Roeper GmbH
- Cargill, Incorporated
- Deosen Biochemical Ltd.
- Devson Impex Private Limited
- DuPont de Nemours, Inc.
- Economy Polymers & Chemicals
- FMC Corporation
- Foodchem International Corporation
- Fufeng Group Company Limited
- Gogia Chemical Industries Pvt. Ltd.
- Hebei Xinhe Biochemical Co., Ltd.
- Hindustan Gum & Chemical Limited
- Ingredion Incorporated
- JM Huber Corporation
- Jungbunzlauer Suisse AG
- Kerry Group plc
- MeiHua Holdings Group Co., Ltd.
- Mitsubishi Corporation
- Salius Pharma Pvt. Ltd.
- Solvay S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
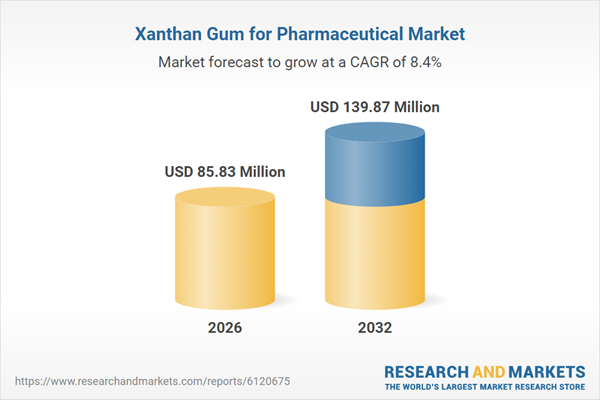
| Estimated Market Value ( USD | $ 85.83 Million |
| Forecasted Market Value ( USD | $ 139.87 Million |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |