Speak directly to the analyst to clarify any post sales queries you may have.
Anti-amyloid-β monoclonal antibodies are reshaping Alzheimer’s care by linking scientific progress to ecosystem readiness, safety governance, and access pathways
Anti-amyloid-β monoclonal antibodies have moved from theoretical promise to a consequential-yet highly scrutinized-therapeutic class in Alzheimer’s disease. Their development has forced the field to reconcile decades of amyloid biology with real-world constraints: how to select patients accurately, how to administer therapy at scale, how to interpret biomarker change versus functional outcomes, and how to manage class-specific safety risks. As a result, the market is no longer defined simply by innovation in antibody engineering; it is shaped by the ability to operationalize diagnosis, monitoring, and risk mitigation in routine clinical settings.What distinguishes this category is the tight coupling between clinical decision-making and system readiness. Treatment initiation often hinges on confirming amyloid pathology, aligning patient expectations with realistic outcomes, and establishing protocols for serial monitoring-especially for amyloid-related imaging abnormalities (ARIA). Consequently, the competitive environment extends beyond drug attributes into the ecosystem that surrounds therapy: infusion capacity, imaging availability, specialist distribution, and payer utilization management.
This executive summary synthesizes the forces reshaping the anti-amyloid-β monoclonal antibody landscape and highlights the commercial implications of evidence evolution, regulatory posture, and healthcare infrastructure. It also outlines the segmentation and regional dynamics that will most influence adoption, then closes with practical recommendations for leaders planning portfolio strategies, partnerships, and go-to-market execution.
Evidence, biomarkers, and care-pathway operationalization are redefining competition as stakeholders demand measurable benefit, scalable delivery, and rigorous safety oversight
The landscape is undergoing transformative shifts driven by three reinforcing dynamics: evidence maturation, operationalization of care pathways, and intensifying scrutiny from regulators and payers. As additional trial readouts, real-world utilization, and post-authorization safety monitoring accumulate, stakeholders are increasingly differentiating between statistical efficacy signals and clinically meaningful, patient-perceived benefit. This has elevated the importance of endpoint selection, subgroup analysis, and longitudinal follow-up, while also raising expectations for transparent communication of risks and uncertainty.In parallel, the field is shifting from “drug-first” thinking to “pathway-first” execution. Anti-amyloid therapy is inseparable from diagnostic confirmation and monitoring infrastructure, making care delivery models a competitive variable. Health systems are building or refining memory-care pathways that integrate neurology, radiology, infusion services, and pharmacy operations. This integration is prompting new norms around pre-treatment workups, MRI schedules, dose titration or interruption protocols, and shared decision-making documentation. Manufacturers that support standardized workflows-without overstepping clinical autonomy-are better positioned to reduce friction at initiation and continuation.
Another major shift is the growing role of biomarkers as both gatekeepers and measures of response. Amyloid PET and cerebrospinal fluid testing remain important, while blood-based biomarkers are progressing rapidly toward broader clinical adoption, particularly as confirmatory tools and triage aids. Even where blood testing does not fully replace confirmatory diagnostics, it can reorganize referral patterns and shorten time-to-treatment. This, in turn, changes where demand concentrates: not only in tertiary centers but also in networks that can reliably coordinate confirmatory testing and infusion logistics.
Finally, stakeholder expectations are evolving toward tighter risk governance. ARIA management is becoming more standardized, including baseline imaging, early-cycle MRI surveillance, and protocols for symptomatic evaluation. This is driving demand for clear labeling interpretation, clinician education, and real-time decision support. As competitors converge on similar scientific narratives, differentiation increasingly comes from evidence credibility, practical implementation support, and the ability to reduce operational burden for providers and payers.
United States tariffs in 2025 may reshape biologics input costs and supply resilience, influencing manufacturing continuity and site-of-care economics for anti-amyloid therapy
United States tariffs in 2025 introduce a layered set of pressures that can affect anti-amyloid-β monoclonal antibodies even when final drug substances are produced domestically. The most immediate exposure tends to sit upstream and adjacent to finished-dose production: single-use bioprocessing components, specialized filtration systems, tubing assemblies, cold-chain packaging, analytical instruments, and certain imaging-related supplies that support diagnosis and monitoring. When tariffs touch these inputs, the impact often appears first as procurement volatility, longer lead times, or forced vendor substitutions rather than as a transparent line-item increase.Because these therapies depend on high-reliability biologics manufacturing, even modest disruptions in qualified supply can cascade into operational risk. Quality agreements, validation status, and change control requirements limit how quickly manufacturers can switch suppliers. Therefore, tariffs can indirectly raise the cost of resilience by pushing companies to dual-source critical components, increase safety stock, or expand domestic qualification efforts. These moves can improve long-term continuity, but they also require near-term capital and organizational focus.
Tariff pressures can also influence provider-facing economics without directly changing list prices. Sites of care may experience higher costs for infusion consumables, pharmacy handling supplies, or imaging operations, especially when equipment service contracts and replacement parts are affected. As health systems absorb or attempt to pass through these costs, they may tighten scheduling, prioritize patients more strictly, or negotiate more assertively with distributors and manufacturers. In a category already dependent on coordinated diagnostics and monitoring, any increase in site-level friction can slow uptake.
Strategically, the most durable response is not reactive cost cutting but structured risk management. Companies that map bill-of-material exposure across geographies, pre-qualify alternative vendors, and integrate tariff scenarios into supply planning can reduce disruption. Just as importantly, cross-functional alignment between manufacturing, access teams, and field support can help ensure that operational changes do not inadvertently undermine adherence to safety monitoring protocols or create variability in patient experience.
Segmentation shows uptake depends on how product design, administration route, distribution pathways, and care settings align with diagnostic confirmation and monitoring demands
Segmentation reveals that adoption patterns are driven less by a single “Alzheimer’s market” and more by the intersection of molecule design, clinical positioning, delivery setting, patient selection, and purchasing pathways. When viewed by product type, fully human and humanized antibodies compete on the practical implications of immunogenicity risk management, tolerability perceptions, and lifecycle optimization, while fragments and engineered variants-where present-invite discussions about tissue penetration, dosing flexibility, and manufacturability. These distinctions matter because they influence how clinicians and payers judge long-term feasibility in a chronic neurodegenerative setting.By application, Alzheimer’s disease dominates strategic focus, yet the clinical reality is that patients arrive with heterogeneous presentations and comorbidities that complicate straightforward categorization. Early symptomatic stages often represent the core target population because they align with trial inclusion norms and monitoring frameworks. However, the “application” lens is increasingly operational: how quickly diagnosis can be confirmed, how reliably follow-up imaging can be completed, and how confidently clinicians can identify patients most likely to benefit while tolerating ARIA surveillance.
Route of administration segmentation underscores why care delivery capacity is central. Intravenous administration concentrates volume in infusion-capable sites and heightens the importance of scheduling efficiency and cold-chain handling. Subcutaneous administration, where available or under development, shifts the access conversation toward broader site participation and potential decentralization, but it does not eliminate the need for diagnostic confirmation and MRI monitoring. In both cases, the route influences staffing, chair time, and patient willingness to persist, which then feeds back into payer assessments of real-world feasibility.
Distribution channel dynamics, spanning hospital pharmacies, retail pharmacies, and online pharmacies, are shaped by how these therapies are dispensed and reimbursed in practice. Hospital and specialty channels tend to dominate when administration is tightly linked to infusion centers and when inventory control and reimbursement support are complex. Retail and online pathways may matter more for ancillary products, patient support logistics, or future formulations that can be handled with less site infrastructure. Ultimately, distribution segmentation is a proxy for how easily therapy can be integrated into existing medication-use systems.
End-user segmentation-hospitals, clinics, and ambulatory surgical centers-captures the operational locus of adoption. Hospitals often anchor diagnosis and monitoring through integrated imaging and specialty neurology services, while clinics can become throughput engines when protocols are standardized and referral pipelines mature. Ambulatory surgical centers may participate when infusion services expand into alternative sites, but their role depends on MRI access coordination and clinical governance for ARIA monitoring. Across end users, the most successful models treat therapy as a managed pathway, not an isolated prescription event.
Regional readiness varies widely as reimbursement models, biomarker access, imaging capacity, and specialist availability determine how quickly anti-amyloid therapy can scale
Regional dynamics reflect the uneven readiness of healthcare systems to deliver a therapy class that depends on specialized diagnosis and serial monitoring. In the Americas, adoption is strongly influenced by payer policies, site-of-care capacity, and the availability of imaging and specialist networks. The region’s innovation density supports rapid pathway formation in leading centers, while broader scaling depends on how efficiently community referral networks can access confirmatory testing and follow-up MRI schedules.Across Europe, Middle East & Africa, heterogeneity is the defining feature. Variation in reimbursement frameworks, centralized health technology assessment expectations, and diagnostic infrastructure creates country-by-country differences in how quickly anti-amyloid therapy can move from specialist centers into wider practice. In several markets, the pace of adoption hinges on the ability to standardize eligibility criteria, fund biomarker confirmation, and maintain consistent safety monitoring. Additionally, workforce constraints in neurology and radiology can become a practical limiter even when policy support exists.
In Asia-Pacific, growth potential is closely linked to expanding diagnostic capacity and investment in specialized care delivery. Some markets combine strong tertiary hospitals with fast-evolving regulatory and reimbursement systems, enabling focused adoption in urban centers. Others face larger gaps in imaging access and specialist density, which can delay broad deployment despite rising disease awareness. Across the region, the trajectory often depends on whether blood-based biomarker testing becomes a scalable front door to diagnosis, allowing earlier identification and more efficient triage into confirmatory pathways.
Taken together, regional segmentation highlights a consistent theme: clinical evidence may be global, but implementation is local. Companies that adapt medical education, site enablement, and access strategies to regional infrastructure-rather than relying on a single global playbook-are better positioned to convert interest into sustained utilization.
Leading companies compete on evidence credibility, ARIA risk enablement, diagnostic partnerships, and supply reliability that make complex Alzheimer’s pathways workable
Company strategies in anti-amyloid-β monoclonal antibodies increasingly converge around three arenas: evidence leadership, safety and monitoring enablement, and ecosystem partnerships. The most influential players build credibility not only through pivotal trials, but through ongoing data generation that clarifies who benefits most, how outcomes translate into day-to-day function, and how risk can be managed consistently across diverse care settings. Real-world evidence programs, pragmatic registries, and post-authorization commitments have become central to sustaining confidence among clinicians, payers, and health systems.Differentiation is also emerging through how companies operationalize ARIA risk governance. Leaders invest in clinician-facing protocols, imaging coordination support, and educational resources that standardize decision-making without oversimplifying clinical nuance. Because ARIA management often requires rapid interpretation of symptoms, MRI findings, and dose decisions, companies that help sites reduce ambiguity can improve continuity of therapy and reduce avoidable discontinuations.
Partnership behavior is another hallmark of leading organizations. Collaborations with diagnostic firms, imaging networks, and health systems can lower barriers to confirming amyloid pathology and completing monitoring on schedule. At the same time, manufacturing and supply chain excellence is becoming a competitive requirement rather than a back-office function. Robust cold-chain logistics, dependable fill-finish capacity, and contingency planning for critical inputs matter deeply in a category where missed infusions or delayed monitoring can undermine both outcomes and confidence.
Finally, companies are refining stakeholder engagement models to match the reality that Alzheimer’s care involves families, caregivers, and multidisciplinary teams. Patient support services, caregiver education, and tools that facilitate shared decision-making can influence persistence and satisfaction, particularly when expectations are aligned to achievable goals. As competition intensifies, the most durable advantage may come from trust built through transparent communication, consistent supply, and practical support that fits clinical workflows.
Leaders can win by scaling diagnosis-to-infusion pathways, operationalizing ARIA safety, building tariff-ready supply resilience, and communicating value with rigor
Industry leaders should prioritize pathway scalability as a first-class strategic objective. That means investing in solutions that shorten time from cognitive concern to confirmed amyloid status, including partnerships that expand access to validated biomarker testing and streamline referrals into specialty care. In parallel, organizations should co-develop site-ready operational playbooks with health systems that cover scheduling, infusion operations, MRI coordination, and documentation standards, enabling consistent delivery across both academic centers and high-throughput community networks.Next, leaders should treat safety management as a value driver rather than a compliance task. Standardized ARIA education, clear escalation pathways for symptoms, and practical guidance for dose interruption or resumption can reduce variability in care. Companies should also align medical and access teams to ensure that utilization management requirements do not unintentionally disrupt monitoring adherence. When payer policies and site protocols are aligned, providers experience less friction and patients are more likely to persist.
Supply resilience must be strengthened in anticipation of trade, tariff, and logistics volatility. Executives should map critical dependencies across single-use components, packaging, analytical supplies, and cold-chain logistics, then prioritize dual sourcing and qualification plans for the highest-risk items. At the same time, leaders should create communication protocols that rapidly inform sites about supply or labeling updates, minimizing last-minute cancellations that erode confidence.
Finally, organizations should refine value communication with disciplined realism. Messaging that clearly explains expected benefits, uncertainties, and monitoring requirements supports shared decision-making and reduces discontinuation driven by misaligned expectations. Over time, the companies that win will be those that integrate scientific leadership with operational humility-building systems that help clinicians deliver therapy safely and predictably at scale.
A triangulated methodology blends clinical, regulatory, operational, and stakeholder inputs to translate anti-amyloid science into implementation-focused insight
The research methodology integrates structured secondary research, expert-informed primary validation, and systematic synthesis to ensure a balanced view of the anti-amyloid-β monoclonal antibody environment. Secondary research emphasizes regulatory actions, clinical trial disclosures, peer-reviewed publications, scientific conference materials, company communications, and healthcare policy documentation. This foundation is used to map the therapeutic class, clarify mechanism-of-action nuances, and identify the operational requirements that shape real-world deployment.Primary research is used to validate assumptions and capture on-the-ground perspectives from stakeholders involved in Alzheimer’s care pathways and biologics commercialization. Interviews and consultations typically span clinicians involved in diagnosis and treatment, pharmacy and infusion operations leaders, payer and reimbursement specialists, and supply chain or manufacturing experts. These discussions focus on practical barriers such as imaging capacity, patient selection, monitoring adherence, and the real implications of site-of-care constraints.
Analytical synthesis applies triangulation across sources to reduce bias and reconcile inconsistencies. Findings are organized around the categories most relevant to executive decisions, including therapeutic differentiation, diagnostic and monitoring ecosystems, reimbursement friction points, and operational readiness. Quality control steps emphasize internal consistency checks, terminology standardization, and careful separation of verified facts from interpretive insights.
Throughout, the methodology is designed to support decision-makers who need more than scientific summaries. It emphasizes how clinical, regulatory, and operational realities interact-because, in this market, execution capability is inseparable from product strategy.
The path to durable anti-amyloid adoption depends on aligning evidence, safety monitoring, supply resilience, and localized care delivery models across stakeholders
Anti-amyloid-β monoclonal antibodies represent a pivotal evolution in Alzheimer’s therapeutics, but their success depends on far more than molecular performance. The category’s trajectory is being shaped by evidence maturation, biomarker-driven diagnosis, and the operational demands of serial monitoring and infusion delivery. As stakeholders raise expectations for transparency, safety governance, and real-world feasibility, companies must compete on credibility and execution simultaneously.Tariff-related pressures in 2025 add another layer of complexity by challenging supply continuity and elevating the value of resilience planning. Meanwhile, segmentation across product type, application, route of administration, distribution channel, and end user clarifies that adoption will concentrate where pathways are integrated and friction is lowest. Regional insights reinforce that infrastructure and policy determine speed of scaling, making localized strategy essential.
The organizations best positioned for durable impact will be those that treat Alzheimer’s therapy as a coordinated service model-linking diagnosis, treatment, monitoring, and patient support into a consistent experience. With disciplined strategy and operational readiness, the class can move closer to its promise of altering the course of disease for appropriately selected patients.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Anti-amyloid-ß Monoclonal Antibodies Market
Companies Mentioned
The key companies profiled in this Anti-amyloid-β Monoclonal Antibodies market report include:- AbbVie Inc.
- Amgen Inc.
- AstraZeneca PLC
- Biogen Inc.
- Bristol Myers Squibb Company
- Eisai Co. Ltd.
- Eli Lilly and Company
- Gilead Sciences Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
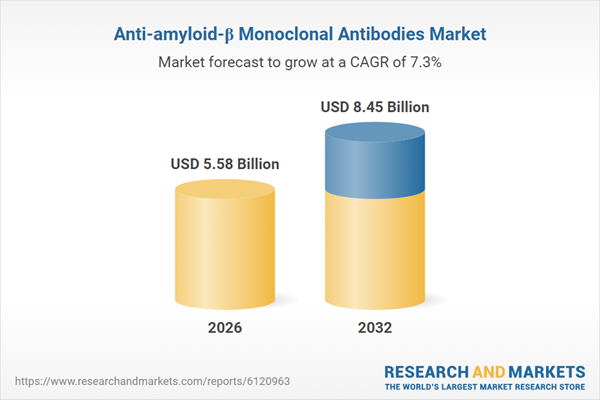
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 5.58 Billion |
| Forecasted Market Value ( USD | $ 8.45 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |