Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to how advanced lid technologies are reshaping clinical packaging, supply chain expectations, and integration with laboratory automation
The medical die cut lids segment is at the confluence of material science, regulatory rigour, and evolving clinical workflows. As sterile packaging becomes ever more central to laboratory throughput and diagnostic accuracy, lid technologies have moved from commodity items to engineered components that influence sample integrity, automation compatibility, and handling ergonomics. Design choices such as peel strength, seal reliability, and compatibility with barcoding or automation interfaces now directly affect downstream laboratory efficiency and compliance with aseptic handling protocols.Recent shifts in manufacturing practice emphasize cleanroom production, validated sealing processes, and traceability across the supply chain. These shifts reflect rising expectations from clinical labs and pharmaceutical manufacturers for consistent performance under varied storage and processing conditions. Consequently, procurement and quality teams are coordinating earlier with product engineers to specify lid attributes that align with instrument platforms, storage temperature ranges, and single-use strategies.
Transitioning from design intent to commercial reality demands closer alignment among material scientists, regulatory affairs specialists, and contract manufacturers. This alignment is accelerating iterative prototyping cycles and prompting investments in tooling flexibility to accommodate smaller batch sizes without compromising validation requirements. For organizations operating in this space, these dynamics underscore the need for cross-functional roadmaps that integrate product performance, regulatory submissions, and scalable manufacturing practices.
Analysis of converging technological advancements, sustainability priorities, and supply chain realignments that are redefining product development and sourcing strategies in the sector
The landscape for medical die cut lids is undergoing transformative shifts driven by several converging forces. First, the push for higher automation and robotic handling in laboratories is increasing demand for lids engineered for consistent mechanical engagement, predictable peel profiles, and low particulate generation. As a result, product development pipelines are prioritizing repeatable mechanical properties and compatibility with automated decapping systems.Second, material innovation is influencing product roadmaps. Advances in polymer formulations, composite laminates, and silicone elastomers are enabling lids that combine robust sealing performance with enhanced biocompatibility and reduced extractables. These material gains are being married to manufacturing process controls that improve batch-to-batch consistency and support regulatory documentation.
Third, sustainability considerations are prompting design reconsiderations. Stakeholders are exploring recyclable substrates, mono-material constructions, and reductions in excess packaging while maintaining sterility and barrier performance. In parallel, suppliers are adopting life cycle thinking to quantify environmental trade-offs and communicating these through product technical files and supplier due diligence programs.
Finally, geopolitical dynamics and evolving trade policies are reshaping sourcing strategies. Companies are diversifying supplier bases, investing in nearshoring, and establishing multi-tier supplier visibility to mitigate disruption. Collectively, these shifts are not isolated; they interact to create a market environment where agility, technical depth, and supply chain transparency are decisive capabilities for market participants.
Comprehensive appraisal of tariff-driven procurement pressures, supplier diversification measures, and operational adjustments reshaping sourcing and innovation choices
Policy changes affecting cross-border trade have immediate consequences for materials procurement, tooling logistics, and manufacturing footprint decisions. When tariffs or similar measures are implemented, they often create direct input cost pressures that ripple through procurement, contract manufacturing agreements, and pricing strategies. In response, buyers and manufacturers frequently evaluate alternative materials, adjust bill-of-materials sourcing, or re-negotiate long-term supplier terms to preserve product performance while controlling cost escalation.In parallel, tariffs tend to accelerate conversations about production localization and supplier diversification. Organizations may prioritize qualification of domestic suppliers, invest in dual-sourcing strategies, or consolidate demand to strategic partners able to absorb policy-related volatility. These actions improve resilience but require upfront investments in supplier audits, process validation, and potential tooling replication.
Operationally, tariffs can extend lead times if suppliers adapt production locations or if logistics patterns change. Procurement and program management teams typically respond by increasing inventory buffers for critical components, refining demand forecasting, and enhancing supplier performance metrics to capture early signals of disruption. Regulatory teams may also need to update technical files to reflect material substitutions or new manufacturing sites, which can introduce additional project timelines.
Finally, tariffs influence innovation pathways. Companies facing higher import costs may accelerate investments in higher-value differentiated features, emphasizing product performance and service to offset pricing pressures. In the current environment, stakeholders balancing cost, compliance, and continuity are prioritizing integrated strategies that combine supply chain redesign, materials engineering, and commercial negotiation to preserve product integrity and customer satisfaction.
Deep segmentation analysis tying product families, end‑use demands, material selection, and distribution approaches to design validation and commercialization priorities
Segmentation insights reveal differentiated needs and innovation vectors across product families and end uses that materially influence R&D priorities and go-to-market strategies. Based on Product Type, design requirements split clearly between Peelable Lids that prioritize controlled peel strength for single-use openings, Permanent Seal Lids where lifelong barrier performance matters, Pressure Sensitive Lids that require precise adhesion profiles, and Resealable Lids engineered for repeated access while maintaining contamination control. These product distinctions inform tooling investments and testing protocols, as peel curves, adhesion aging, and reseal cycles require distinct validation methods.Based on End Use, performance specifications are tailored to application environments: Diagnostic Containers need rapid access and clear biocontainment characteristics, Laboratory Plates demand uniform sealing across arrays compatible with automated thermal cyclers, and Pharmaceutical Vials require robust barrier properties and compatibility with lyophilization or cold storage. Understanding these end-use drivers supports targeted material selection and process validation pathways that optimize for both usability and regulatory evidence.
Based on Material, formulation choices shape both functional and regulatory outcomes. Aluminum continues to offer high barrier performance for critical vial applications, composites provide engineered barrier layers and structural attributes, plastics present low-weight, customizable solutions with the Plastic category further subdivided into Polyethylene, Polypropylene, and PTFE to address chemical resistance, thermal performance, and low-extractable requirements, while Silicone materials bring soft-seal properties and elastic recovery beneficial for resealable designs. Each material class demands specific equipment, contamination control practices, and biocompatibility testing.
Based on Distribution Channel, commercialization strategies vary with channel dynamics. Direct Sales models favor tailored contract work and tight collaboration with OEMs and end users, Distributors broaden geographic reach and provide localized inventory, and Online Retail enables rapid access for smaller buyers and specialty applications. These channels shape inventory policies, lead-time commitments, and the structure of post-sale technical support. Integrating these segmentation lenses provides a cohesive understanding of where investments in design, quality systems, and commercial coverage will deliver the greatest strategic returns.
Regional operational and regulatory contrasts that determine sourcing priorities, compliance prerequisites, and commercialization pathways across global markets
Regional dynamics create distinct competitive and operational considerations that affect sourcing, regulatory strategy, and route-to-market choices. In the Americas, emphasis remains on regulatory compliance, quality system maturity, and integration with high-throughput laboratories. Supplier relationships in this region often emphasize rapid technical support, validated manufacturing in controlled environments, and the capacity to meet stringent documentation requirements for clinical and pharmaceutical customers.In Europe, Middle East & Africa, the landscape is shaped by harmonized regulatory frameworks, sustainability regulations, and a mix of localized manufacturing hubs. Companies operating here typically invest in extended product technical files, environmental declarations, and regional distribution partnerships to address diverse market access requirements and to reduce cross-border friction. This region also demonstrates strong interest in circularity initiatives and extended producer responsibility measures that influence material choices and packaging design.
Asia-Pacific exhibits a combination of advanced manufacturing capability and rapidly growing clinical and diagnostic demand. The region is a focal point for tooling capacity and material processing expertise, while also presenting a wide range of regulatory pathways across national jurisdictions. Manufacturers and buyers interacting with Asia-Pacific partners focus on supplier qualification protocols, export control considerations, and collaborative quality improvement programs to ensure consistency across global supply chains.
Across regions, a common thread is the need for clear traceability and alignment between commercial, quality, and regulatory functions. Firms that proactively align regional sourcing strategies with compliance requirements and customer expectations tend to navigate complexities more effectively and reduce time-to-market friction for new lid designs.
Competitive landscape insights highlighting engineering differentiation, analytical capability investments, and supply chain architectures that create durable customer advantage
Competitive dynamics in the sector reflect a blend of engineering differentiation, service delivery, and supply chain resilience. Leading organizations emphasize cross-disciplinary engineering teams that marry materials expertise with manufacturability, enabling faster iteration between prototyping and validated production runs. This approach reduces the time from concept validation to commercial availability while maintaining rigorous documentation required by clinical and pharmaceutical customers.Strategic investments in in-house analytical capabilities, including extractables and leachables testing, peel profile analysis, and accelerated aging chambers, provide suppliers with defensible performance claims and shorten qualification timelines for customers. Companies that combine these capabilities with scalable cleanroom production and validated sterilization pathways gain a competitive edge when serving regulated end markets.
Supply chain strategies also distinguish market leaders. Firms adopting multi-region manufacturing footprints, standardized quality management systems, and digital supplier performance dashboards typically demonstrate higher responsiveness to disruption and more predictable lead times. In addition, providers offering value-added services such as kitting, customized labeling, and integrated inventory management create stronger procurement stickiness with OEMs and laboratories.
Finally, collaboration models matter. Strategic partnerships with material suppliers, contract manufacturers, and instrumentation OEMs accelerate feature integration and ensure that lid designs are co-developed to meet automation and analytical instrument requirements. Organizations that foster collaborative innovation while maintaining strict quality governance position themselves as preferred suppliers for complex, high-value applications.
Actionable strategic priorities for product, supply chain, and commercial leaders to build resilient, differentiated, and customer‑centric offerings in the lid market
Leaders should prioritize a tri-fold strategy that balances technical differentiation, supply chain resilience, and customer-aligned commercialization. First, invest in material science and analytical validation capabilities to substantiate performance claims and enable faster qualification by clinical and pharmaceutical buyers. Strengthened laboratory testing and documented stability evidence will reduce procurement friction and support product positioning for sensitive applications.Second, develop a resilient supplier ecosystem through dual-sourcing of critical substrates, targeted nearshoring where feasible, and qualification programs that include environmental and social governance assessments. Enhancing supplier transparency and modularizing tooling can shorten response times and lower risk exposure to geopolitical or policy shifts.
Third, align commercial channels with customer needs by combining direct technical engagement for OEM relationships, distributor partnerships for geographic reach, and online platforms for rapid fulfillment of specialized or small-batch orders. Bundling value-added services such as kitting, labeling, and just-in-time replenishment will deepen client relationships and increase switching costs.
Additionally, embed sustainability into product roadmaps by evaluating mono-material designs, recyclable substrates, and reduced material use without compromising barrier properties. Simultaneously, maintain rigorous regulatory alignment by updating technical documentation promptly when materials or manufacturing sites change. Executed together, these actions will strengthen market positioning, reduce operational fragility, and support profitable growth in a changing policy and technology landscape.
Transparent methodology combining primary stakeholder interviews, technical literature review, and scenario analysis to produce validated practical insights for decision-makers
This research synthesis integrates primary interviews with procurement leaders, quality managers, and R&D engineers alongside a structured review of technical literature and publicly available regulatory guidance. The analysis places equal weight on operational practices observed across manufacturing sites and documented performance characteristics reported in product technical files. Primary qualitative insights were gathered through in-depth discussions with stakeholders across manufacturing, clinical, and distribution functions to capture practical challenges and decision criteria.Secondary research comprised a targeted review of material science publications, industry white papers, and regulatory guidance documents relevant to sterile packaging, extractables and leachables, and quality system expectations. Where appropriate, findings were triangulated across multiple sources to validate recurring themes and to identify divergence in regional practices. Throughout the process, emphasis was placed on traceability of sources and clarity about the context in which specific practices are applied.
Analytical approaches included comparative capability mapping, segmentation crosswalks to align product attributes with end-use requirements, and scenario analysis to evaluate supply chain responses to policy shifts. The methodology intentionally avoided reliance on proprietary market databases in favor of corroborated qualitative evidence and technical reviews. This approach yields practical, implementable insights for decision-makers focused on product quality, supply continuity, and regulatory alignment.
Concluding synthesis emphasizing the shift from commodity procurement to engineered component strategies that drive resilience, differentiation, and customer trust
In conclusion, the medical die cut lids domain is evolving from standardized commodity procurement into a differentiated engineering space where material selection, process validation, and supply chain architecture determine commercial outcomes. As automation and sustainability considerations rise in prominence, successful participants will be those that pair material and analytical expertise with agile manufacturing and clear regulatory documentation. These capabilities reduce time-to-deployment for new lid designs and enhance trust with clinical and pharmaceutical customers.Policy shifts and regional dynamics require a dynamic sourcing playbook that balances near-term continuity with long-term strategic investments. Firms that proactively qualify alternative suppliers, standardize quality systems across sites, and invest in value-added commercial services will mitigate risk and preserve customer relationships. At the same time, aligning product roadmaps with sustainability goals and instrument compatibility will create meaningful differentiation in procurement conversations.
Looking ahead, the most resilient organizations will treat lids as engineered components within broader diagnostic and pharmaceutical ecosystems. This mindset shift-from commodity lens to integrated component strategy-unlocks opportunities for premium positioning, deeper OEM partnerships, and more predictable operational performance across fluctuating policy and supply environments.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Medical Die Cut Lids Market
Companies Mentioned
The key companies profiled in this Medical Die Cut Lids market report include:- Ahlstrom-Munksjö Oyj
- Amcor PLC
- AptarGroup, Inc.
- Berry Global Group, Inc.
- Freudenberg SE
- Gerresheimer AG
- Lohmann GmbH & Co. KG
- Rogers Corporation
- Sealed Air Corporation
- Technipaq, Inc.
- Uflex Ltd.
- West Pharmaceutical Services, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
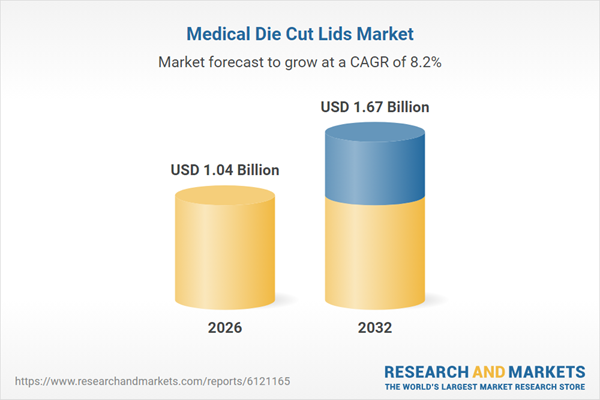
| Estimated Market Value ( USD | $ 1.04 Billion |
| Forecasted Market Value ( USD | $ 1.67 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |