Speak directly to the analyst to clarify any post sales queries you may have.
Postbiotics emerge as stability-first microbiome actives, reshaping how brands deliver gut, immune, and skin benefits with credible science and scalable manufacturing
Postbiotics have moved from a niche scientific concept to a pragmatic product strategy for brands that want microbiome-related benefits without the fragility and cold-chain constraints commonly associated with live microbes. As non-viable microbial cells, cell fragments, and metabolites that can confer health or functional benefits, postbiotics are increasingly positioned as a stability-forward alternative that fits modern manufacturing realities. This shift is occurring as consumers and professionals alike demand more credible, better-tolerated, and easier-to-formulate solutions for gut comfort, immune resilience, skin health, and metabolic wellbeing.At the same time, the category is being shaped by an intersection of regulatory scrutiny, formulation advances, and a more educated consumer who expects clarity on mechanisms rather than vague wellness promises. Brands are learning that postbiotics are not a single ingredient type but a family of materials with distinct production methods, bioactive profiles, and quality-control needs. Consequently, competitive advantage is increasingly earned through transparent characterization, consistent sourcing, and disciplined clinical substantiation.
Moreover, postbiotics are benefiting from broader momentum in microbiome science and the mainstream acceptance of fermented foods, synbiotics, and functional nutrition. As a result, postbiotics are showing up in multiple product architectures, from capsules and sachets to beverages and topical skincare, with messaging that emphasizes reliability, shelf stability, and compatibility with sensitive consumers. This executive summary synthesizes the most important landscape shifts, policy impacts, segmentation and regional dynamics, competitive themes, and strategic actions for leaders seeking to build durable positions in postbiotics.
The category shifts from probiotic-adjacent messaging to reproducible bioactives, higher substantiation standards, and stability-led innovation across products and channels
The postbiotics landscape is undergoing a decisive transition from ingredient curiosity to outcomes-driven platforms built around reproducibility and regulatory defensibility. Previously, many product concepts borrowed the language of probiotics without fully differentiating what “inactivated” or “non-viable” truly means. Now, the industry is converging on tighter identity specifications, improved analytical methods, and more explicit discussions of what bioactive fractions are present and why they matter. This evolution is making it easier for formulators to design products with predictable performance, but it is also raising the bar for documentation and quality.In parallel, brand strategies are shifting toward formats that reduce operational risk. Postbiotics can be integrated into dry blends, heat-processed foods, and shelf-stable beverages more readily than live cultures, which is changing innovation roadmaps for both consumer packaged goods and supplements. This is particularly transformative in omnichannel retail, where ambient shipping and longer dwell times can degrade sensitive ingredients. By emphasizing stability and consistency, postbiotics help brands protect consumer experience across e-commerce and traditional retail.
Scientific storytelling is also changing. Rather than broadly claiming “supports gut health,” leading companies are mapping specific outcomes to specific bioactives and pathways, such as barrier function support, modulation of inflammatory signaling, or skin microbiome balancing. This is driving a more sophisticated approach to substantiation, including well-defined ingredient characterization, human-use data where feasible, and robust safety and allergen considerations. Consequently, postbiotics are beginning to behave like an advanced functional ingredient category rather than a generalized wellness trend.
Finally, the ecosystem is becoming more partnership-centric. Ingredient suppliers are investing in proprietary strains and controlled inactivation processes, while consumer brands seek turnkey dossiers, claim language guidance, and formulation support. As this collaboration deepens, differentiation is increasingly anchored in manufacturing know-how, traceability, and the ability to deliver consistent lots at scale-especially important as policy and trade conditions reshape supply availability and costs.
Tariff dynamics in the United States reshape postbiotics sourcing, landed-cost engineering, and packaging decisions, elevating supply assurance as a core strategy lever
United States tariff conditions in 2025 are influencing postbiotics supply chains in ways that go beyond simple price changes, particularly because many postbiotic inputs, processing aids, and packaging components traverse multiple borders before becoming finished goods. As companies diversify sourcing and evaluate nearshoring, postbiotics benefit from their shelf-stable nature, which can reduce reliance on expedited shipping and cold-chain services. However, tariff-driven cost pressure can still surface in fermentation inputs, specialized filtration media, encapsulation materials, and contract manufacturing services tied to imported equipment or subcomponents.In response, procurement teams are renegotiating contracts with a sharper focus on total landed cost, customs classification discipline, and supplier transparency. This is pushing manufacturers to document origin, processing steps, and value-add locations more rigorously to avoid surprises at entry. It is also accelerating the use of multi-sourcing strategies for critical inputs, including carbohydrate feedstocks and excipients used in powder stabilization. For brands, this translates into a more prominent role for supply assurance in innovation decisions, with some product teams selecting postbiotic formats specifically because they are easier to buffer against logistics volatility.
Tariffs are also prompting a redesign of packaging and fill-finish decisions. When packaging components or certain polymers face cost spikes, companies are more likely to standardize bottle sizes, reduce SKU complexity, and prioritize flexible packaging or simplified labels to contain costs. Importantly, these operational shifts can cascade into brand strategy: streamlined assortments encourage clearer hero claims, fewer overlapping variants, and more deliberate channel segmentation.
Over the same period, heightened policy attention to health-related products increases the value of conservative claim strategies and strong compliance documentation. While tariffs are not a regulatory approval mechanism, they often coincide with broader scrutiny of cross-border trade in dietary supplements and functional ingredients. Consequently, the most resilient players are those who pair tariff-aware sourcing with robust quality systems, ensuring that cost optimization does not undermine consistency, safety, or claim credibility.
Segmentation clarifies how postbiotic type, composition, format, application, end-user needs, and channel strategy interact to determine winning positioning and product design
Segmentation reveals that postbiotics strategies diverge meaningfully depending on product type, source and composition, delivery format, application focus, end user, and route to market, making disciplined positioning essential. Within product type, heat-inactivated cells, microbial lysates, and purified metabolites each carry different formulation behaviors and storytelling advantages; as a result, brands that clarify which type they use and why can more easily communicate benefit logic and quality expectations. Source and composition choices further differentiate offerings, since bacterial-derived materials often support gut and immune narratives while yeast-derived fractions frequently align with skin and barrier-focused positioning, and mixed compositions introduce both opportunity and complexity in standardization.Delivery format is becoming a decisive battleground because it shapes consumer experience, manufacturing risk, and channel fit. Capsules and tablets remain favored where dosage precision and claim conservatism are priorities, while powders and stick packs enable daily-use rituals and bundling with fibers, minerals, or flavors. Meanwhile, functional foods and ready-to-drink concepts are gaining attention because postbiotics can better withstand processing and distribution than live cultures, enabling broader retail placement and easier global expansion. This format diversification is also encouraging brands to rethink sensorial design, as off-notes or texture changes from bioactive fractions must be managed through masking, microencapsulation, and excipient selection.
Application segmentation highlights how the category is moving toward more targeted benefit platforms. Digestive comfort and immune support remain central, but skin health, women’s health, pediatric tolerance, and metabolic wellbeing are increasingly prominent as brands seek less crowded claim territory and higher consumer willingness to pay for specificity. In parallel, clinical and professional channels often prioritize gut barrier function and symptom-adjacent support narratives, while mass channels lean toward everyday resilience and “gentle” wellness framing. These differences matter because they dictate the depth of substantiation required and the risk tolerance for more assertive language.
End-user and channel segmentation reinforce that adoption is not uniform. Adult general wellness remains foundational, but formulations tailored for seniors, athletes, and sensitive consumers are rising as companies emphasize tolerability and predictable performance. On routes to market, e-commerce and direct-to-consumer models favor education-heavy storytelling and subscription replenishment, whereas pharmacies and specialty retail reward clarity, recognizable science cues, and conservative compliance posture. Taken together, segmentation indicates that winners will treat postbiotics not as a single SKU idea but as a portfolio architecture-matching ingredient type, format, and channel to a coherent benefit promise and a credible evidence plan.
Regional insights reveal how regulation, consumer expectations, and retail models across the Americas, Europe, Middle East & Africa, and Asia-Pacific shape adoption paths
Regional dynamics show that postbiotics adoption reflects differences in regulatory expectations, consumer health priorities, and retail structures, creating distinct playbooks for each geography. In the Americas, commercialization momentum is closely tied to dietary supplement innovation, practitioner influence, and omnichannel retail expansion, with growing emphasis on transparent labeling and quality documentation. Brands operating here increasingly compete on trust signals such as batch testing rigor, clear definitions of what constitutes the postbiotic material, and conservative claim framing that can scale across multiple states and retail partners.Across Europe, the landscape is shaped by strong expectations for scientific substantiation and careful language around health benefits, which encourages companies to invest early in compliant communication and standardized ingredient characterization. This environment often favors suppliers and brand owners who can demonstrate reproducible production, robust safety assessment, and clear traceability. As a result, postbiotics in Europe frequently lean into precision and professional endorsement, with a measured approach to consumer-facing claims that prioritizes credibility over hype.
In the Middle East and Africa, market development varies widely by country, but common themes include increasing interest in digestive wellness, rising modern retail penetration in key urban centers, and a growing appetite for premium functional products. Supply chain reliability and shelf stability are particularly valuable in this region, and postbiotics can offer a pragmatic pathway to microbiome-oriented innovation without the distribution constraints of live cultures. Education remains pivotal, as consumers and retailers often require clearer explanations of what postbiotics are and how they differ from probiotics.
Asia-Pacific stands out for its strong cultural familiarity with fermentation, rapid functional food innovation, and high receptivity to beauty-from-within and skin health narratives. At the same time, the region’s diversity in regulatory regimes and consumer preferences demands localized formulation and messaging strategies. Companies that align postbiotic concepts with established wellness traditions while maintaining modern scientific credibility are better positioned to scale, especially as digital commerce and cross-border purchasing continue to influence discovery and trial.
Company strategies converge on proprietary processes, standardized postbiotic profiles, and partnership-led innovation that converts microbiome science into scalable product advantages
Competitive behavior in postbiotics is increasingly defined by proprietary production processes, ingredient standardization, and the ability to translate complex microbiome science into brand-ready claims. Leading ingredient companies are differentiating through controlled fermentation, validated inactivation steps, and consistent profiling of key bioactive fractions, recognizing that buyers want more than a generic “ferment” descriptor. This is driving investments in quality systems, analytical capabilities, and documentation packages that shorten product development cycles for brand partners.At the brand level, companies are choosing between two dominant approaches: platform building versus hero-ingredient integration. Platform builders develop cohesive lines around a consistent postbiotic identity, often pairing it with prebiotics or select botanicals to create a broader gut-skin-immune narrative. In contrast, integrators introduce postbiotics as an upgrade to existing SKUs-positioning them as gentler, more stable, or more reliable than live cultures-while keeping the broader brand promise unchanged. Both approaches can win, but each requires disciplined alignment between evidence, labeling, and consumer education.
Partnership models are also evolving. Contract manufacturers are expanding capabilities for spray drying, microencapsulation, and blending that preserves bioactivity while improving flowability and taste. Meanwhile, retailers and distributors increasingly expect clear substantiation and supply continuity, which elevates suppliers that can offer redundant manufacturing, robust stability data, and transparent specifications. As scrutiny increases, companies with strong governance around strain provenance, allergen management, and contaminant controls are better positioned to secure long-term listings and professional recommendations.
Finally, innovation is moving beyond supplements into adjacent categories such as functional beverages, medical nutrition-style products, and dermocosmetic formulations. This convergence is widening the competitive set to include food and personal care incumbents, raising expectations for sensorial excellence, claims discipline, and brand storytelling that resonates across multiple consumer touchpoints.
Actionable moves center on defensible ingredient identity, mechanism-aligned claims, formulation excellence, tariff-resilient operations, and evidence roadmaps that scale
Industry leaders can strengthen their postbiotics position by first tightening ingredient identity and verification. Selecting materials with clear definitions-what organism is used, how it is inactivated, which fractions are standardized, and how stability is validated-reduces downstream compliance risk and improves consumer trust. In parallel, teams should design claims around mechanisms and use contexts they can defend, prioritizing language that aligns with labeling rules in target markets and can remain consistent across channels.Next, leaders should treat formulation and sensorial performance as strategic differentiators rather than technical afterthoughts. Postbiotics may be stable, but they still interact with flavors, acids, sweeteners, proteins, and emulsifiers, especially in ready-to-drink and functional food applications. Investing early in pilot testing, compatibility screening, and packaging trials can prevent late-stage reformulation that delays launches. Where taste masking or microencapsulation is required, partnering with specialists can shorten development timelines and protect brand reputation.
Operationally, tariff-aware supply planning should become a standard part of the innovation gate process. Dual sourcing for key inputs, disciplined customs documentation, and thoughtful decisions about domestic versus offshore processing can reduce volatility in margin and availability. Additionally, simplifying SKU architecture and harmonizing packaging components can improve resilience while keeping shelf presence strong.
Finally, leaders should build an evidence roadmap that matches ambition to feasibility. This may include leveraging existing human-use data where appropriate, investing in targeted studies for priority claims, and adopting real-world feedback loops through post-launch monitoring. When combined with strong education-explaining what postbiotics are, why non-viable does not mean non-functional, and how to use the product consistently-these actions can create a defensible, scalable growth strategy.
A rigorous methodology combines scientific and regulatory review with value-chain interviews and triangulation to validate commercial realities and strategic priorities
This research methodology integrates systematic secondary research with structured primary engagement to ensure a balanced, decision-useful view of the postbiotics product landscape. Secondary research focuses on scientific literature, regulatory guidance, patent activity, company technical materials, product labels, and public filings to map ingredient definitions, production approaches, application trends, and compliance considerations. This foundation helps establish consistent terminology and clarifies how postbiotics are characterized across product categories.Primary research is conducted through interviews and consultations with stakeholders across the value chain, including ingredient manufacturers, contract manufacturers, brand owners, distributors, clinicians or practitioners where relevant, and retail decision-makers. These discussions are designed to validate practical constraints and success factors such as stability expectations, preferred formats, claim sensitivities, quality documentation needs, and common formulation challenges. Insights from these engagements are synthesized to identify recurring themes and points of divergence between regions, channels, and application areas.
Data triangulation is used throughout the process to cross-validate findings and reduce bias. When discrepancies arise-such as differing definitions of postbiotic materials or conflicting views on preferred claims-inputs are reconciled by examining underlying assumptions, reviewing technical documentation, and prioritizing the most current and jurisdiction-relevant guidance. Quality assurance steps include consistency checks, terminology normalization, and peer review to ensure that conclusions are coherent, traceable to evidence, and actionable for strategy teams.
The result is a methodology built to support real-world decisions: selecting ingredient partners, shaping compliant messaging, designing product formats, and preparing for operational risks such as tariff-driven supply shocks. By combining scientific grounding with commercial validation, the research aims to reflect how postbiotics are being developed, evaluated, and adopted in practice.
Postbiotics advance from trend to durable platform as standards tighten, supply resilience matters more, and targeted positioning separates leaders from followers
Postbiotics are becoming a core innovation pathway for companies that want microbiome-related benefits delivered with stability, scalability, and clearer manufacturing control. As the category matures, competitive advantage is shifting toward those who can define what they use, prove it is consistent, and communicate benefits in a way that aligns with regulatory expectations and consumer understanding.Landscape shifts point to greater standardization, tighter substantiation norms, and deeper collaboration between ingredient suppliers, manufacturers, and brands. Meanwhile, United States tariff conditions in 2025 amplify the importance of supply assurance and landed-cost discipline, making operational readiness a strategic differentiator rather than a back-office concern.
Segmentation and regional insights reinforce that there is no single winning playbook. Success depends on aligning ingredient type, format, application, and channel strategy with the expectations of each region and the evidence required for the chosen positioning. Companies that execute this alignment-while investing in formulation performance and education-are best positioned to build durable trust and long-term category leadership.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Postbiotics Product Market
Companies Mentioned
The key companies profiled in this Postbiotics Product market report include:- Archer Daniels Midland Company
- BASF SE
- Beekeeper's Naturals, Inc.
- BioGaia AB
- Cargill, Incorporated
- Chr. Hansen Holding A/S
- CJ CheilJedang Corp.
- Danone S.A.
- Dr Emil Nutrition
- DSM-Firmenich AG
- Essential Formulas, Inc.
- Glac Biotech Co., Ltd.
- Kerry Group plc
- Kirin Holdings Company, Limited
- Lallemand Inc.
- Lesaffre S.A.
- Lonza Group Ltd.
- Morinaga Milk Industry Co., Ltd.
- Nestlé S.A.
- Postbiotica S.r.l.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
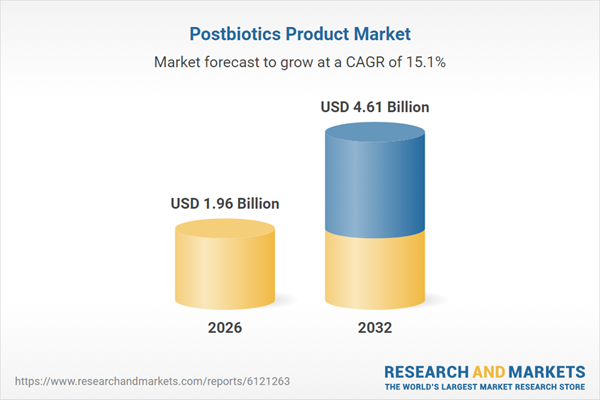
| Estimated Market Value ( USD | $ 1.96 Billion |
| Forecasted Market Value ( USD | $ 4.61 Billion |
| Compound Annual Growth Rate | 15.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |