Speak directly to the analyst to clarify any post sales queries you may have.
Highly active universal nuclease demand is accelerating as bioprocessing and advanced analytics prioritize speed, reproducibility, and validated performance
Highly active universal nucleases have become foundational tools for modern life science workflows because they compress timelines while improving sample quality across diverse matrices. As sequencing, cell therapy, and advanced biologics manufacturing scale, laboratories and production teams increasingly depend on enzymes that can rapidly and reliably remove unwanted nucleic acids without compromising target integrity. This class of nuclease is valued for its broad substrate scope, high catalytic efficiency, and compatibility with streamlined protocols, enabling consistent outcomes in both research and regulated environments.What makes the current moment distinctive is how universal nucleases now sit at the intersection of scientific performance and operational resilience. End users are no longer evaluating only speed and activity; they are assessing lot-to-lot consistency, impurity profiles, traceability, and supplier reliability. At the same time, automation-first laboratories and digital quality systems are raising expectations for reproducibility, standardized documentation, and ready-to-integrate formats.
Against this backdrop, competitive differentiation is shifting toward application-fit engineering and end-to-end support. Suppliers that can pair enzyme performance with clear validation packages, flexible formats, and dependable fulfillment are becoming preferred partners. Consequently, the market narrative is expanding from “a powerful nuclease” to “a validated, workflow-ready component” that reduces risk, labor, and variability at scale.
The nuclease ecosystem is being reshaped by workflow consolidation, automation readiness, and compliance-driven purchasing in regulated life sciences
The landscape for highly active universal nucleases is undergoing several transformative shifts driven by how life science work is executed today. First, workflows are consolidating around fewer, more versatile enzymes to simplify procurement, reduce training burden, and improve standardization across sites. Universal nucleases benefit from this consolidation because they can replace multiple specialty reagents, particularly where teams want a single validated solution spanning discovery, development, and manufacturing.In parallel, enzyme engineering and formulation science are reshaping expectations for “universal” performance. End users increasingly require activity across challenging buffers, temperature ranges, and sample types, including high-protein lysates, viscous bioprocess intermediates, and complex clinical matrices. This is fueling innovation in stabilizers, lyophilized or room-temperature formats, and nuclease variants tuned for reduced inhibition or improved compatibility with downstream enzymes.
Another pivotal shift is the rise of automation and high-throughput operations. Liquid-handling platforms and integrated sample-to-answer systems reward reagents that deliver consistent kinetics, minimal foaming, and predictable reaction completion. As a result, suppliers are investing in format options such as pre-aliquoted tubes, plates, and bulk packs designed for robotics, along with documentation that supports method transfer between instruments and sites.
Finally, quality and compliance expectations are tightening as nucleases move deeper into regulated manufacturing and clinical laboratories. Buyers are placing greater emphasis on traceable raw materials, controlled bioburden, residual impurity characterization, and robust certificates of analysis. This shift is also changing commercial engagement: technical support now extends beyond troubleshooting into co-development, protocol harmonization, and audit-ready documentation packages that reduce friction during validation and scale-up.
United States tariff pressure in 2025 may reshape nuclease sourcing through higher input costs, supplier qualification urgency, and localized fulfillment
United States tariff actions anticipated for 2025 are expected to influence procurement strategies and supply-chain design for enzyme-based reagents, including highly active universal nucleases. Even when tariffs do not directly target finished enzymes, the cumulative impact can propagate through upstream inputs such as fermentation media components, chromatography resins, single-use plastics, cold-chain packaging, and specialized lab consumables. As these costs ripple across suppliers, buyers may face changes in lead times, pricing structures, and minimum order constraints.In response, many organizations are likely to increase dual sourcing and regionalize critical supply where feasible. For nuclease suppliers, this can translate into greater demand for domestically finished or domestically packaged products, expansion of local warehousing, and more transparent documentation of country of origin for key inputs. For end users, particularly those in biomanufacturing, the emphasis will be on minimizing production disruptions by qualifying alternates early and incorporating tariff sensitivity into vendor risk assessments.
Tariff-related uncertainty also elevates the strategic value of inventory planning and contract structures. Longer-term agreements with defined pricing corridors, safety-stock programs, and flexible delivery schedules can reduce exposure to sudden cost escalations or logistics bottlenecks. At the same time, organizations may revisit make-versus-buy decisions for certain reagents, especially where volume is predictable and internal quality systems can support enzyme qualification.
Over time, these dynamics can accelerate investment in localized manufacturing capacity and encourage deeper collaboration between suppliers and large-volume customers. The practical outcome is a purchasing environment where technical performance remains essential, but resilience, traceability, and total landed cost increasingly shape which nuclease products are selected and how supply relationships are managed.
Segmentation dynamics highlight how product formats, validation depth, and end-user workflows determine which nuclease solutions win adoption
Segmentation patterns for highly active universal nucleases reveal a market defined as much by workflow requirements as by enzyme chemistry. Across product type expectations, buyers differentiate between broad-spectrum nucleases optimized for rapid degradation and variants positioned for gentler processing where downstream sensitivity is a concern. This distinction becomes especially important when teams are balancing aggressive nucleic acid removal against preserving protein complexes, viral vectors, or other labile targets.From a format and packaging perspective, purchasing decisions increasingly reflect operational scale. Small-volume research settings prioritize convenience, stability, and minimal waste, whereas industrial and core laboratory environments demand bulk formats, consistent kinetics across lots, and integration with automated dispensing. These requirements also influence preferences for liquid versus lyophilized presentations, with stability and shipping constraints shaping adoption in distributed lab networks.
Application-driven segmentation shows the most decisive differences in validation depth and documentation needs. In research workflows, performance is often judged by time-to-result and compatibility with common buffers. In contrast, bioprocess and clinical-adjacent applications require rigorous impurity profiles, traceability, and reproducible performance under defined operating windows. The closer a nuclease is to a regulated release or a validated manufacturing step, the more weight buyers place on documentation packages, change-control communication, and audit readiness.
End-user segmentation further highlights how procurement pathways diverge. Academic and early-stage teams may accept broader tolerance in packaging or lot strategy, while large biopharma and contract development and manufacturing organizations place a premium on supply assurance, technical support depth, and predictable lifecycle management. Distribution and purchasing channels also shape outcomes, since some organizations prefer direct technical engagement for qualification, while others rely on approved vendor catalogs to accelerate ordering.
Taken together, these segmentation insights underscore a core theme: successful products are not simply high-activity enzymes; they are application-fit solutions tailored to how different buyers validate, deploy, and scale nuclease-dependent processes.
Regional adoption diverges by biomanufacturing scale, regulatory rigor, and logistics readiness across the Americas, EMEA, and Asia-Pacific
Regional dynamics for highly active universal nucleases reflect differences in biomanufacturing intensity, research funding structures, regulatory expectations, and supply-chain maturity. In the Americas, strong demand is tied to advanced biologics development, large-scale bioprocessing capacity, and fast adoption of automation, all of which elevate expectations for documentation, supply continuity, and lot-to-lot reproducibility. Buyers in this region also tend to formalize supplier qualification early, especially when nucleases support critical process steps.Across Europe, Middle East, and Africa, the purchasing landscape is shaped by a combination of robust academic research ecosystems, growing bioprocess footprints, and stringent quality expectations. Demand is reinforced by the region’s emphasis on standardized laboratory practices and quality management, which can favor suppliers that provide clear validation support and consistent product lifecycle communication. Additionally, cross-border logistics considerations make local warehousing and stable cold-chain execution meaningful differentiators.
In Asia-Pacific, expansion in biotechnology manufacturing, increasing adoption of high-throughput molecular methods, and a rapidly professionalizing supplier ecosystem are driving broader uptake. As capacity grows, many buyers prioritize scalability, cost-performance balance, and dependable lead times, while also seeking products that align with evolving regulatory and quality frameworks. The region’s diversity creates multiple demand centers, with established hubs pushing for premium validation and emerging markets emphasizing accessibility and operational simplicity.
Overall, regional insight points to a shared direction: as nuclease use spreads from research into manufacturing and clinical-adjacent workflows, the competitive baseline rises everywhere. Suppliers that can combine high activity with localized service models, resilient logistics, and region-appropriate documentation are best positioned to capture sustained adoption.
Competitive advantage is shifting toward validated performance, workflow-centric portfolios, and audit-ready quality systems backed by resilient supply models
Company strategies in the highly active universal nuclease space increasingly converge on three differentiators: performance credibility, workflow integration, and supply assurance. Leading suppliers emphasize enzyme activity backed by reproducible kinetics, robust stability data, and application notes that translate performance claims into practical protocols. This focus reduces evaluation time for end users and supports faster internal alignment across R&D, quality, and procurement teams.A second theme is portfolio design around end-to-end workflows rather than standalone reagents. Companies are positioning nucleases alongside complementary solutions such as sample preparation kits, buffer systems, and downstream cleanup tools, enabling customers to standardize processes and reduce variability. This approach is especially relevant for high-throughput labs and bioprocess environments where reducing touchpoints and method complexity can materially improve throughput and compliance.
Third, suppliers are strengthening quality systems and transparency to meet regulated use cases. This includes more comprehensive certificates of analysis, clearer statements on residual impurities, and tighter change-control practices. Many companies also invest in regional distribution capabilities, cold-chain robustness, and redundancy in critical inputs to maintain continuity during geopolitical or logistics disruptions.
Competitive intensity is also shaped by collaboration models. Beyond traditional selling, companies differentiate through technical partnerships, co-validation projects, and support for method transfer into automated platforms. As a result, the strongest positions are increasingly held by suppliers that can operate as long-term partners-supporting qualification, scaling, and ongoing optimization-rather than as commodity enzyme providers.
Actionable steps focus on qualification rigor, supply resilience, and workflow standardization to reduce risk while improving operational throughput
Industry leaders can strengthen their position by treating highly active universal nucleases as strategic process components rather than interchangeable reagents. The first priority is to align nuclease selection criteria with the criticality of the workflow. For regulated or near-regulated steps, teams should define acceptance criteria that include impurity thresholds, traceability expectations, and change-control requirements, then map those criteria to supplier documentation before initiating full-scale qualification.Next, organizations should proactively engineer supply resilience. Qualifying at least one alternative product early, negotiating inventory or safety-stock arrangements, and clarifying country-of-origin exposure for upstream inputs can reduce disruption risk. Where tariffs or logistics volatility is expected, leaders should incorporate total landed cost, lead-time variability, and substitution feasibility into sourcing decisions rather than relying on unit price alone.
Operational excellence is another lever. Standardizing nuclease protocols across sites, building automation-compatible methods, and documenting reaction completion criteria can reduce operator variability and rework. Leaders should also evaluate format choices through the lens of waste reduction and contamination control, particularly in high-throughput settings where packaging and handling materially affect throughput.
Finally, R&D and commercial teams should collaborate on application-fit differentiation. Suppliers and product owners can prioritize development of formulations that tolerate inhibitors, enable room-temperature shipping where scientifically justified, or integrate cleanly into closed or single-use systems. By linking product design to real workflow pain points, organizations can improve customer retention, reduce validation friction, and accelerate adoption across multiple use cases.
Methodology combines stakeholder interviews, technical documentation review, and triangulation to convert complex enzyme criteria into clear decisions
The research methodology for assessing the highly active universal nuclease landscape is structured to translate technical realities into decision-ready business insight. It begins with a rigorous definition of scope, including product categories, use cases, and the value chain from raw inputs through manufacturing, packaging, and distribution. This framing ensures that comparisons reflect how nucleases are actually evaluated and deployed in research and production settings.Primary research emphasizes interviews and structured inputs from stakeholders across the ecosystem, including manufacturers, distributors, laboratory managers, bioprocess engineers, and quality professionals. These perspectives are used to understand purchasing criteria, validation practices, pain points in deployment, and emerging requirements tied to automation and compliance. Feedback is systematically cross-checked to distinguish common patterns from outlier experiences.
Secondary research complements these inputs through review of publicly available technical documentation, regulatory guidance themes relevant to enzyme use in controlled environments, product literature, and corporate communications. The goal is to validate claims regarding formats, quality practices, and application positioning without relying on unverifiable assertions.
Finally, triangulation is applied to reconcile differing viewpoints and ensure internal consistency across findings. Throughout the process, emphasis is placed on clarity, traceability of assumptions, and separation of observed trends from interpretive conclusions. This approach supports readers who must justify procurement, development, and partnership decisions in environments where both scientific performance and operational risk matter.
Universal nucleases are becoming critical workflow infrastructure as performance, compliance, and supply resilience converge into a single buying priority
Highly active universal nucleases are moving from optional efficiency enhancers to essential enablers of modern life science execution. Their role is expanding as organizations push for faster turnaround, higher throughput, and tighter reproducibility across both exploratory research and scaled manufacturing. As this shift continues, buyers will increasingly reward solutions that are not only powerful but also predictable, well-documented, and easy to integrate into automated and standardized workflows.Meanwhile, external pressures such as tariff-driven cost variability and logistics uncertainty are reinforcing a broader industry pivot toward resilient sourcing and transparent supplier practices. These factors amplify the importance of proactive qualification strategies, regional fulfillment capabilities, and clear lifecycle management.
Ultimately, success in this landscape will favor organizations that connect enzyme performance to operational outcomes. Suppliers that design for workflow fit and compliance readiness, and buyers that evaluate nucleases through the lens of total risk and process robustness, will be best positioned to sustain efficiency gains while protecting quality and continuity.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Highly Active Universal Nuclease Market
Companies Mentioned
The key companies profiled in this Highly Active Universal Nuclease market report include:- Agilent Technologies, Inc.
- Applied Biosystems LLC
- Assay Biotechnology Company
- Becton, Dickinson and Company (BD)
- Bio‑Rad Laboratories, Inc.
- Enzymatics Inc.
- F. Hoffmann‑La Roche Ltd.
- GE Healthcare Life Sciences
- Lucigen Corporation
- Merck KGaA
- New England Biolabs, Inc.
- New England BioProducts Ltd.
- Promega Corporation
- Qiagen N.V.
- Sartorius AG
- Takara Bio Inc.
- Takara Shuzo Co., Ltd.
- Thermo Fisher Scientific Inc.
- Worthington Biochemical Corporation
- Zymo Research Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
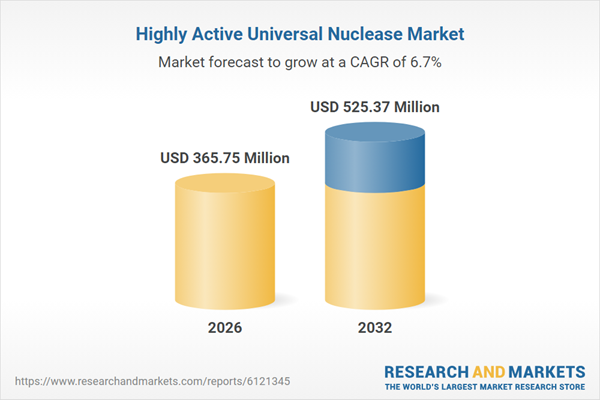
| Estimated Market Value ( USD | $ 365.75 Million |
| Forecasted Market Value ( USD | $ 525.37 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |