Speak directly to the analyst to clarify any post sales queries you may have.
Nitinol compression staples are redefining fixation expectations by pairing material-driven compression with streamlined workflow and rising clinical performance demands
Nitinol compression staples have moved from being a niche fixation option to a strategically important solution set across multiple orthopedic and trauma use cases. Their appeal is rooted in the material science of nickel-titanium alloys: superelasticity and shape memory enable sustained compressive forces across osteotomy or fracture sites, supporting stable apposition while accommodating micro-motion that can be beneficial for bone healing. As procedural expectations rise, these devices are increasingly positioned not merely as “another implant,” but as a workflow and outcomes lever-reducing the variability associated with manual compression techniques and enabling more consistent fixation constructs.At the same time, surgeons and hospital buyers are evaluating nitinol staples within a broader shift toward minimally disruptive fixation and reproducibility. Nitinol compression staples can shorten steps in the operative sequence, reduce the need for additional fixation in selected indications, and potentially limit soft-tissue disruption compared with bulkier constructs. These advantages resonate in foot and ankle surgery, hand and wrist procedures, and certain pediatric and deformity correction contexts where anatomy is constrained and compression is critical.
This executive summary synthesizes how technology evolution, clinical evidence expectations, procurement dynamics, and policy pressures are reshaping the competitive arena for nitinol compression staples. It frames what is changing, why it matters now, and how decision-makers can respond with targeted product, market access, and operational choices that fit today’s orthopedic value equation.
Outpatient migration, evidence-driven purchasing, and design-for-workflow innovation are reshaping how nitinol compression staples compete and win adoption
The landscape for nitinol compression staples is being reshaped by a convergence of clinical, operational, and manufacturing shifts that are broader than any single product cycle. First, orthopedic care delivery is continuing its migration toward outpatient and short-stay environments. In these settings, predictable operative time, reduced instrumentation complexity, and consistent fixation behavior become more valuable. Compression staples align with this preference because they can reduce reliance on intraoperative manual compression maneuvers and can simplify construct planning in selected cases.Second, there is a visible shift in what “evidence” means for adoption. Surgeons still value tactile feedback and personal experience, but hospital committees increasingly expect indication-specific data, clarity on revision pathways, and compatibility with standardized care protocols. This is pushing manufacturers to invest in comparative studies, post-market surveillance rigor, and clearer education on staple sizing, placement technique, and metallurgy-driven behavior under cyclic loading.
Third, innovation is increasingly centered on design refinements rather than only expanding SKU counts. Companies are focusing on leg geometry for improved purchase, bridge profiles to manage soft-tissue irritation, and insertion tools that reduce malalignment risk. Coatings and surface treatments are also under scrutiny as stakeholders ask how to balance osseointegration potential with removability and artifact considerations in imaging.
Fourth, procurement behavior is shifting toward contract rationalization and kit-based purchasing. Integrated offerings-staples plus dedicated instrumentation, compatible drivers, and standardized trays-can win preference when they reduce sterilization burden and variability across service lines. This dynamic elevates the importance of service reliability, rep support, and training models that work for both high-volume centers and geographically dispersed outpatient facilities.
Finally, supply-chain resilience and compliance expectations are rising. Traceability requirements, tighter quality-system audits, and scrutiny of nickel-related sensitivities are influencing labeling, risk management documentation, and customer communication. As a result, competitive advantage is increasingly defined by manufacturing consistency and the ability to support customers with rapid answers, not just by the device’s compressive force profile.
United States tariffs in 2025 intensify supply-chain and contracting pressures, making resilience, validation agility, and value justification central to staple adoption
The cumulative impact of United States tariffs in 2025 is best understood through how it compounds existing pressures on the orthopedic implant supply chain rather than through any single cost line item. Nitinol staples rely on specialized inputs, precision machining, heat treatment, and validation steps that can span multiple countries. When tariffs raise the landed cost of components, packaging materials, or capital equipment used in manufacturing and inspection, companies face a choice between margin compression, selective price adjustments, or redesigning the sourcing footprint.In practice, the most immediate effect often shows up in procurement negotiations and contracting cadence. Hospitals and ambulatory surgery centers are already balancing cost containment with surgeon preference items. Tariff-driven increases can make renewals more contentious, especially for staples positioned as premium solutions. This tends to accelerate value justification requirements, pushing suppliers to demonstrate how compression staples can reduce operative steps, lower instrument count, or improve consistency in ways that matter to administrators as well as clinicians.
Over time, tariffs can also influence inventory strategy. To avoid disruption, some suppliers may increase safety stock of key components or finished goods, but that ties up working capital and raises the importance of demand planning accuracy. Others may shift to dual-sourcing or nearshoring for certain operations, which can improve resilience but requires revalidation and careful regulatory documentation. The result is a more complex operational environment where quality, continuity, and total landed cost are tightly coupled.
Moreover, tariffs may indirectly shape innovation timelines. When engineering teams are pulled into supplier qualification, material substitutions, or packaging redesigns to mitigate tariff exposure, fewer resources remain for new product development. The companies that navigate 2025 effectively will be those that treat tariff response as a cross-functional program-integrating regulatory, quality, finance, and commercial teams-so the customer experience remains stable even as the supply chain evolves.
Segmentation shows adoption is shaped by procedure-specific compression goals, site-of-care economics, and design choices that balance purchase, profile, and reliability
Segmentation reveals that demand patterns for nitinol compression staples are not uniform; they are shaped by how clinical objectives, purchasing pathways, and procedural settings intersect. When viewed by product type, the market differentiates between staple designs optimized for small-bone fixation and those engineered for higher-load applications, with geometry, bridge profile, and insertion tooling becoming meaningful decision criteria. In parallel, size and configuration choices influence surgeon preference, as the ability to match staple footprint to anatomy can determine whether compression is achieved without compromising surrounding soft tissue.Looking through the lens of application, staples are evaluated differently in forefoot, midfoot, hindfoot, and ankle procedures than they are in hand, wrist, elbow, or cranio-maxillofacial contexts. In foot and ankle surgery, particularly for arthrodesis and osteotomy, sustained compression and low-profile constructs are repeatedly emphasized, while in upper extremity procedures, precision placement and minimizing hardware prominence can be decisive. Trauma and deformity correction scenarios introduce yet another set of expectations, including construct stability under cyclic load, compatibility with adjunct fixation, and predictable behavior in compromised bone.
End-user segmentation further clarifies adoption drivers. Hospitals often prioritize standardization, vendor reliability, and the ability to support multiple surgeons with consistent trays and training. Ambulatory surgery centers, by contrast, may focus more heavily on operative efficiency, reduced instrument burden, and streamlined logistics that fit high-turnover schedules. Specialty orthopedic clinics can sit between these extremes, valuing both surgeon autonomy and operational simplicity, particularly when procedures are performed across multiple affiliated sites.
Material and manufacturing-related segmentation considerations also matter, even when customers do not frame them explicitly as such. Differences in nitinol processing, heat-treatment protocols, and quality controls can influence staple deployment behavior and compression consistency. As buyers become more sophisticated, they increasingly ask for clarity on performance under repeated loading, MRI considerations, and the practical pathway for removal if revision is needed. These segmentation-aligned needs suggest that winning strategies are those that tailor clinical education, tray configuration, and contracting models to how each segment defines “predictable fixation.”
Regional adoption differs by outpatient penetration, tendering intensity, and distributor maturity, making localized access and support models decisive for growth
Regional dynamics for nitinol compression staples reflect differences in care pathways, regulatory expectations, and procurement models, and these differences shape how suppliers should prioritize commercialization. In the Americas, demand is strongly influenced by outpatient growth and contracting discipline, with decision-making often balancing surgeon preference against system-level standardization. Clinical education and rep support remain pivotal, but so does the ability to document operational value in addition to clinical performance.Across Europe, Middle East, and Africa, diversity in reimbursement environments and hospital purchasing structures creates a more heterogeneous adoption curve. In some European markets, structured tendering and cost-effectiveness narratives carry substantial weight, while certain specialty centers prioritize innovative fixation solutions for complex reconstructions. The need for multilingual training, region-specific regulatory compliance, and consistent supply can be a differentiator, especially when hospital groups seek to rationalize vendors across networks.
In Asia-Pacific, growth is often linked to expanding orthopedic capacity, increasing sub-specialization, and investment in surgical infrastructure. However, market entry and scaling can be shaped by country-specific regulatory timelines, local distribution capabilities, and the degree to which premium implants are embraced within public versus private systems. As surgeon training ecosystems strengthen and ambulatory models expand in select countries, staples positioned as efficient, reproducible fixation tools can gain traction-particularly when supported by strong local clinical engagement and dependable instrument logistics.
Across regions, one consistent theme is the rising expectation of continuity: customers want assurance that staples and compatible instruments will remain available, supported, and serviceable over time. Consequently, regional success increasingly depends on aligning the go-to-market model-distribution, inventory placement, training cadence, and tender strategy-to local procurement realities rather than relying on a single global playbook.
Winning companies pair surgeon-trusted design with manufacturing discipline, service reliability, and compelling procedural solutions that withstand committee scrutiny
Competition in nitinol compression staples is shaped by the interplay of engineering credibility, surgeon trust, and operational execution. Established orthopedic companies benefit from broad relationships, integrated instrument ecosystems, and contracting leverage, allowing staples to be positioned as part of a cohesive procedural solution rather than a standalone implant. These players often emphasize tray standardization, cross-selling through adjacent fixation portfolios, and clinical education platforms that can scale across hospital systems.Specialized companies and focused innovators compete by moving faster on design iteration and by tailoring staple options to specific procedure families. They may differentiate through insertion tools that reduce placement variability, through staple geometries designed to optimize purchase in small bones, or through portfolios that address niche indications where large incumbents have limited depth. In many cases, their success hinges on field support quality and on building surgeon champions who can translate technical advantages into practical preference.
Across both groups, differentiation is increasingly tied to manufacturing consistency and documentation readiness. Customers are asking harder questions about lot-to-lot behavior, the durability of compression under cyclic loading, and the predictability of deployment across different bone qualities. Companies that can articulate their nitinol processing controls, quality assurance checkpoints, and real-world performance monitoring are better positioned when value analysis committees and supply-chain teams are involved.
Partnerships also matter. Collaborations with distributors, instrument service providers, and training organizations can extend reach, particularly in regions where direct sales coverage is limited. Meanwhile, the ability to support hospitals with rapid replenishment, instrument maintenance, and clear revision or removal pathways can convert trial usage into sustained adoption, especially in service lines where consistency and turnover time define profitability.
Leaders can outpace competition by aligning indication focus, evidence, and supply resilience while delivering standardized kits and training that reduce OR variability
Industry leaders can strengthen their position by treating nitinol compression staples as an integrated solution rather than a commodity implant. Start by sharpening indication focus: prioritize procedure areas where sustained compression and low-profile fixation clearly address unmet needs, and align clinical messaging to the specific outcomes surgeons and administrators care about in those cases. As you refine the story, ensure the economic narrative is equally concrete, connecting staple use to predictable workflow, reduced variability, and instrument efficiency.Next, invest in executional excellence across the customer journey. Standardize tray configurations to reduce confusion, simplify reordering, and support multi-surgeon environments, while maintaining enough flexibility to accommodate anatomical variability. Strengthen surgeon training with technique-focused education that addresses staple sizing, placement angles, and bone-quality considerations, and supplement this with operating room staff training to minimize setup and sterilization friction. Where outpatient sites are expanding, adapt support models to match their faster turnover and leaner staffing.
On the operations side, proactively mitigate trade and supply-chain risk. Develop dual-sourcing strategies for vulnerable inputs, validate alternate suppliers before disruptions occur, and build a tariff response plan that includes regulatory and quality stakeholders from the outset. Transparently communicate availability and lead times to customers, because reliability can be as persuasive as innovation when hospitals are standardizing.
Finally, elevate evidence generation and post-market learning. Establish registries or real-world data programs with clear governance, prioritize comparative studies where clinical uncertainty limits adoption, and translate findings into practical guidance rather than abstract claims. By linking design choices to measurable procedural consistency and by demonstrating readiness for procurement scrutiny, leaders can protect price integrity while expanding appropriate utilization.
A triangulated methodology blending stakeholder interviews, regulatory and clinical review, and structured synthesis delivers decision-grade insights without overreliance on any single input
The research methodology for this report combines structured primary engagement with rigorous secondary review to build a decision-grade view of the nitinol compression staples landscape. The process begins by defining the product scope and clinical boundaries, clarifying which staple designs and procedure families are included, and mapping the ecosystem of stakeholders who influence adoption, including surgeons, operating room leaders, procurement teams, and distribution partners.Primary research draws on interviews and structured discussions with knowledgeable participants across the value chain. These engagements are designed to surface how devices are selected, what performance attributes are prioritized in different procedures, how training and instrumentation affect adoption, and where supply-chain reliability influences contracting. Insights are triangulated across roles to reduce single-perspective bias, and findings are continuously checked for internal consistency.
Secondary research includes review of publicly available regulatory information, company communications, clinical literature, standards guidance relevant to implantable materials, and broader policy developments affecting medical device trade and compliance. This desk research supports validation of terminology, identification of competitive positioning themes, and confirmation of market dynamics without relying on any single narrative.
Finally, the analysis phase applies a structured framework to synthesize insights into coherent conclusions. Segmentation and regional lenses are used to organize adoption drivers, while competitive assessment focuses on differentiation factors such as design, instrumentation, evidence posture, and operational execution. Throughout, the emphasis remains on producing practical, strategy-oriented insights that can inform product planning, commercial prioritization, and risk management decisions.
Nitinol compression staples are moving from optional fixation to standardized solutions as outpatient care, procurement scrutiny, and supply resilience redefine success
Nitinol compression staples are increasingly evaluated as a pathway to more reproducible fixation, particularly where anatomy is constrained and consistent compression is difficult to achieve with traditional techniques. As outpatient care expands and hospitals standardize, the bar for adoption is rising: stakeholders want clear procedural benefits, dependable supply, and evidence that aligns with both clinical goals and operational realities.At the same time, external pressures such as tariff-related cost volatility and supply-chain complexity are forcing manufacturers to compete on resilience and responsiveness as much as on design. Companies that can maintain stable availability, support efficient instrumentation workflows, and communicate performance with clarity are better positioned to earn trust from surgeons and procurement teams alike.
Looking ahead, the most durable advantage will come from aligning product engineering, clinical education, and operational excellence to the specific needs of each procedure segment and region. Those who treat staples as part of a broader procedural solution-supported by credible data and reliable service-will be best equipped to convert clinical interest into sustained utilization.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Nitinol Compression Staples Market
Companies Mentioned
The key companies profiled in this Nitinol Compression Staples market report include:- Acumed, LLC
- Alphatec Spine, Inc.
- Arthrex, Inc.
- BioMedical Enterprises, Inc.
- BioPro Implants, Inc.
- ConMed Corporation
- CrossRoads Extremity Systems, Inc.
- Enovis Corporation
- Globus Medical, Inc.
- Groupe Lépine
- Johnson & Johnson
- Medical Component Specialists, Inc.
- Medline UNITE
- Medtronic plc
- Metric Medical Devices, LLC
- MicroPort Scientific Corporation
- Novastep, Inc.
- NuVasive, Inc.
- Nvision Biomedical Technologies, Inc.
- Orthofix Medical Inc.
- Smith & Nephew plc
- Stryker Corporation
- Trax Surgical, LLC
- TriMed, Inc.
- Vilex, Inc.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
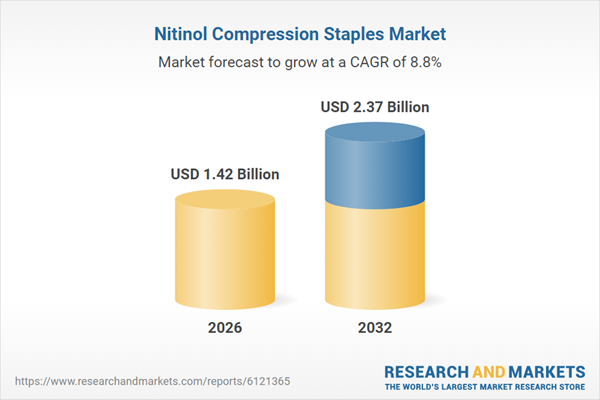
| Estimated Market Value ( USD | $ 1.42 Billion |
| Forecasted Market Value ( USD | $ 2.37 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |