Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to optical biometry devices that connects technological fundamentals with clinical outcomes and evolving deployment patterns across care settings
Optical biometry has evolved from a niche technical capability into an essential clinical tool that supports precise intraocular lens selection and advanced refractive planning. This introduction frames the device landscape by connecting technological principles with clinical outcomes, contextualizing how rapid diagnostic precision and non-invasive measurement have changed surgical workflows. Optical Low Coherence Reflectometry and Partial Coherence Interferometry underpin contemporary devices, enabling repeatable axial length and anterior segment measurements that clinicians rely on to reduce refractive surprises.Beyond the core measurement modalities, recent improvements in software algorithms, enhanced user interfaces, and interoperability with electronic health records have simplified device adoption across care settings. Ambulatory surgical centers, clinics, diagnostic centers, and hospitals increasingly view optical biometry not only as a diagnostic instrument but also as a quality control mechanism that supports faster patient throughput and improved surgical outcomes. Desktop, handheld, and portable deployments address diverse clinical environments, from high-volume surgical suites to outreach programs and low-resource clinics.
As payers and providers emphasize value-based care, the role of optical biometry in improving refractive accuracy and reducing postoperative interventions has become more prominent. The introduction concludes by establishing the device category as a convergence point for optics, software, and workflow integration, setting the stage for a deeper examination of market shifts, tariff impacts, segmentation insights, and regional dynamics.
How advances in sensor design, algorithmic analytics, and care delivery models are reshaping product road maps and procurement expectations in optical biometry
The landscape for optical biometry is experiencing transformative shifts driven by parallel advances in sensor technology, machine learning-driven algorithms, and demands for seamless clinical integration. Optical devices now incorporate higher-resolution interferometric sensors, improved motion compensation, and automated quality metrics that minimize operator dependence and accelerate throughput. These technical refinements have enabled manufacturers to differentiate on speed, ease of use, and measurement reproducibility, prompting a redefinition of product road maps and competitive positioning.Concurrently, software and analytics have become key value drivers. Algorithmic enhancements improve IOL power calculations, enable more accurate lens constant optimization, and facilitate integration with electronic surgical planning tools. This trend has elevated partnerships between device makers and software developers, and it has encouraged acquisition strategies that bring analytics capabilities in-house. Clinicians benefit from streamlined workflows and reduced manual data entry, while procurement teams gain clearer evaluation criteria centered on interoperability and upgrade pathways.
Service delivery models are shifting as well. Ambulatory surgical centers and clinics favor devices that balance clinical performance with space efficiency and ease of maintenance, leading to an expanded emphasis on handheld and portable units for decentralized care. At the same time, hospitals and diagnostic centers continue to invest in high-throughput desktop systems that support large surgical volumes. The convergence of miniaturized optics, cloud-enabled software, and a focus on point-of-care accessibility is reshaping product road maps and altering buyer expectations globally.
Tariff-induced supply chain realignments are prompting nearshoring, regional assembly, and revised procurement strategies to safeguard device availability and service continuity
The imposition of tariffs and related trade measures has introduced a layer of complexity to global supply chains and procurement strategies for optical biometry devices. Manufacturers have responded by reassessing component sourcing, reallocating manufacturing footprints, and negotiating alternative logistics routes to preserve margin and maintain competitive pricing. Procurement teams face longer lead times and increased attention to total landed cost, prompting closer collaboration between clinical engineering, supply chain, and finance functions to ensure uninterrupted device availability.Tariff-driven cost pressures have accelerated conversations about nearshoring and regional assembly hubs, particularly for elective care markets with predictable demand patterns. Vendors that can localize final assembly or source critical subcomponents regionally gain a resilience advantage, reducing tariff exposure and shortening replenishment cycles. At the same time, service and maintenance models are under scrutiny; organizations seek extended warranties, local spares stocking, and remote diagnostic capabilities to mitigate downtime risks associated with cross-border parts delays.
Clinicians and administrators must weigh short-term cost impacts against long-term supplier stability when evaluating capital investments. In some instances, total cost of ownership calculations now incorporate tariff contingencies and higher spare parts inventory. The cumulative effect of these trade measures has elevated supplier diligence, encouraged diversification of sourcing, and shifted negotiation leverage toward vendors with established regional capabilities and responsive after-sales support.
Segmentation-driven insights that align device technologies, clinical applications, end-user realities, deployment choices, and sales channels to adoption dynamics
Segmentation offers a practical lens to assess technology adoption, clinical fit, and commercial pathways. Based on product type, the industry divides between Optical Low Coherence Reflectometry and Partial Coherence Interferometry, each offering distinct trade-offs in measurement principle and device architecture that influence clinician preference and procurement criteria. Based on application, devices are used primarily to support Cataract Surgery and Refractive Surgery, with each clinical use case demanding specific measurement fidelity and integration with surgical planning tools.Based on end user, deployment decisions reflect the operating realities of Ambulatory Surgical Centers, Clinics, Diagnostic Centers, and Hospitals; high-volume surgical centers prioritize throughput and automation while clinics and outreach programs value portability and ease of use. Based on deployment mode, the choice between Desktop, Handheld, and Portable configurations affects space planning, staff training, and maintenance protocols, driving differential purchase rationale across care settings. Based on sales channel, distribution occurs through Channel Partners and Direct Sales, and the Channel Partners route further diversifies into Distributors and Online Retail, creating distinct service expectations, lead times, and pricing structures.
Understanding these layers of segmentation helps stakeholders map product capabilities to clinical workflows, prioritize evaluation criteria, and design commercialization strategies that reflect end-user needs. Transitional considerations such as interoperability, upgradeability, and training support cross-cut segment boundaries, informing longer-term adoption and lifecycle management decisions.
Regional dynamics across the Americas, Europe, Middle East & Africa, and Asia-Pacific that influence device selection, compliance needs, and localized service expectations
Regional dynamics shape technology adoption, reimbursement practices, and procurement priorities in distinct ways across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, private healthcare delivery models and high-volume ambulatory surgery centers drive demand for high-throughput desktop systems and integrated surgical planning ecosystems. Clinician networks and consolidated buying groups influence purchasing cycles, and service expectations emphasize rapid technical support and consumable availability.In Europe, Middle East & Africa, regulatory harmonization in parts of Europe contrasts with heterogeneous reimbursement and procurement practices elsewhere in the region, affecting device selection and deployment timelines. Hospitals and diagnostic centers often seek robust compliance documentation and lifecycle support, while clinics and outreach programs prioritize cost-effective portable solutions to extend access. In Asia-Pacific, rapid expansion of ophthalmic services, a growing middle-class demand for elective procedures, and investment in decentralized care models amplify interest in both portable handheld units for outreach and integrated desktop devices for tertiary centers.
Across all regions, interoperability, training, and after-sales service remain central to adoption. Vendors that can localize support, demonstrate regulatory compliance, and provide scalable service models find greater traction. Transitional regional strategies that prioritize partnerships with local distributors, tailored training programs, and modular product offerings tend to outperform one-size-fits-all approaches in heterogeneous healthcare systems.
Competitive positioning hinges on combining precise measurement hardware, advanced analytics, and scalable service models to win clinical trust and procurement preference
Competitive dynamics in the optical biometry space center on the ability to combine precision hardware with robust software, dependable service frameworks, and scalable commercial models. Leading companies emphasize differentiated measurement accuracy, streamlined user interfaces, and end-to-end interoperability with surgical planning and electronic health record systems. Some organizations pursue inorganic growth to acquire niche software capabilities or to broaden product portfolios, while others focus on iterative hardware improvements paired with long-term service contracts.Strategic partnerships between device OEMs, software vendors, and clinical networks have become more common, accelerating integration pathways and creating bundled value propositions that resonate with high-volume surgical centers. Service excellence-comprising rapid field support, local spares availability, and remote diagnostics-serves as a competitive moat in many markets, especially where tariff-related supply disruptions have increased the perceived value of local responsiveness.
From a commercialization perspective, companies that diversify sales channels by combining direct enterprise sales with channel partner networks, including distributors and online retail options, are better positioned to address heterogeneous buyer journeys. Ultimately, success depends on delivering consistent clinical outcomes, ensuring regulatory compliance across jurisdictions, and providing scalable training and support programs that reduce the burden on clinical staff and accelerate time-to-value.
Actionable steps to strengthen product longevity, supply chain resilience, interoperability, and localized support to accelerate clinical adoption and procurement
Industry leaders should prioritize a set of actionable steps to stabilize supply chains, strengthen clinical partnerships, and accelerate adoption in diverse care settings. First, invest in modular device architectures and software upgradability to extend product lifecycles and enable incremental feature rollouts that preserve installed-base value. This approach reduces capital intensity for buyers and creates revenue streams through upgrade packages and software subscriptions. Second, formalize regional assembly or final configuration capabilities to reduce tariff exposure and improve lead times, which fosters stronger relationships with procurement teams seeking predictable delivery.Third, expand interoperability efforts by offering open APIs, standardized data export formats, and certifiable integrations with the most commonly used electronic health records and surgical planning platforms. Enhanced interoperability lowers adoption barriers and increases device utility across clinical pathways. Fourth, build robust local support networks that combine training academies, certified service partners, and remote diagnostics to minimize downtime and demonstrate a total cost-of-ownership advantage. Fifth, tailor commercial models to end-user needs by offering flexible financing, bundled service agreements, and pilot programs for high-volume centers and outreach initiatives.
Taken together, these measures increase resilience, accelerate clinical adoption, and align commercial strategy with the operational realities of ambulatory surgical centers, clinics, diagnostic centers, and hospitals while addressing the distinct requirements of desktop, handheld, and portable deployments.
Transparent research methodology combining clinician interviews, product feature mapping, and service capability assessments to inform procurement and clinical integration
This research synthesizes primary and secondary intelligence to produce a practitioner-focused analysis of device capabilities, adoption trends, and commercial strategies. Primary inputs include structured interviews with clinicians across ophthalmology subspecialties, procurement leaders from ambulatory surgical centers and hospitals, and technical conversations with product engineers and service managers. Secondary sources encompass peer-reviewed clinical literature, regulatory filings, product technical specifications, and public industry announcements that inform technology comparisons and feature analyses.Analytical methods combine qualitative thematic analysis with comparative device feature mapping and supplier capability assessments. The approach emphasizes triangulation: cross-validating clinician perspectives with product specifications and service performance reports to produce a balanced view of real-world device utility. Attention to deployment contexts-desktop, handheld, and portable-ensures that recommendations address the operational and clinical constraints typical of ambulatory surgical centers, clinics, diagnostic centers, and hospitals.
Data governance includes documented interview protocols, source attribution where permissible, and a reproducible framework for assessing interoperability, service readiness, and clinical integration potential. The methodology aims to be transparent and reproducible, enabling stakeholders to understand the basis for conclusions and to request tailored follow-up analyses aligned to specific procurement or clinical questions.
Conclusion highlighting the convergence of precision optics, analytics, and regional service models as the decisive factors for clinical and commercial success
In conclusion, optical biometry devices are central to modern ophthalmic care, blending optical engineering, software analytics, and service models to improve clinical outcomes and operational efficiency. The evolving landscape favors vendors that can deliver precise, repeatable measurements while simplifying integration with surgical planning workflows and electronic health records. Clinicians and procurement teams will increasingly evaluate devices not only on technical merit but also on interoperable software, regional service strength, and adaptable commercial models that reflect deployment realities across ambulatory surgical centers, clinics, diagnostic centers, and hospitals.Trade-related pressures and supply chain reconfiguration underscore the importance of regionalized assembly, diversified sourcing, and robust after-sales networks. Strategic investments in modular hardware, algorithmic improvements, and scalable support programs position companies to meet the varied needs of desktop, handheld, and portable deployments. Ultimately, the most resilient and commercially successful organizations will align product innovation with pragmatic service delivery, enabling clinicians to deliver better refractive outcomes and administrators to control lifecycle costs while expanding access to quality eye care.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Optical Biometry Devices Market
Companies Mentioned
The key companies profiled in this Optical Biometry Devices market report include:- AMETEK, Inc.
- Bausch + Lomb Corporation
- Carl Zeiss AG
- Centralvue S.p.A.
- Danaher Corporation
- EssilorLuxottica S.A.
- Haag-Streit AG
- Heine Optotechnik GmbH & Co. KG
- Hoya Corporation
- MEDA Co., Ltd.
- NIDEK Co., Ltd.
- Optovue, Inc.
- Revenio Group Corporation
- Santec Corporation
- Tomey Corporation
- Topcon Corporation
- Visionix Ltd.
- Ziemer Ophthalmic Systems AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
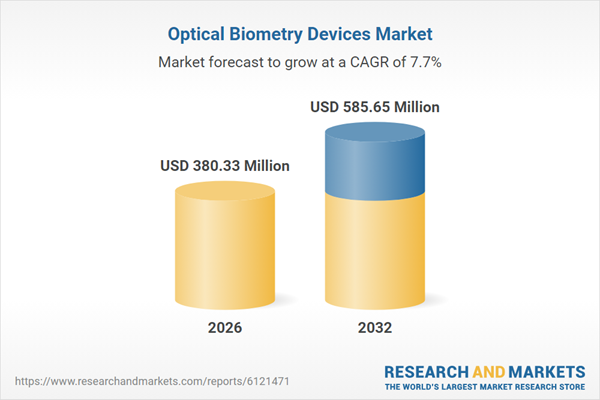
| Estimated Market Value ( USD | $ 380.33 Million |
| Forecasted Market Value ( USD | $ 585.65 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |