Speak directly to the analyst to clarify any post sales queries you may have.
Intraoperative nerve monitoring is evolving from optional adjunct to core surgical safeguard, reshaping expectations for performance, workflow integration, and value
Intraoperative nerve monitoring systems sit at the intersection of surgical precision, patient safety, and operating room efficiency. As procedure complexity increases across spine, ENT, thyroid, vascular, and select neurosurgical interventions, the tolerance for avoidable nerve injury continues to narrow. Hospitals and surgical teams increasingly view nerve monitoring not as an optional adjunct, but as a practical safeguard that can support better functional outcomes, reduce post-operative complications, and strengthen documentation of intraoperative decision-making.At the same time, the category is evolving beyond the traditional model of standalone monitoring boxes paired with basic electrodes. Clinical expectations now encompass consistent signal quality, streamlined workflow integration, and the ability to tailor modalities to patient-specific risk profiles. This is pushing vendors to innovate in hardware design, electrode offerings, and software-driven signal interpretation while also addressing training, service models, and interoperability.
As the landscape matures, decision-makers face a more nuanced set of trade-offs. They must evaluate performance and reliability under real operating conditions, the availability of specialized support, and the long-term cost of ownership across disposables, upgrades, and maintenance. In that context, the executive perspective must connect clinical value to procurement realities and operational adoption, setting the stage for how this market is being reshaped by technology, policy, and shifting care delivery models.
Workflow integration, software-led differentiation, and evolving service models are redefining how intraoperative nerve monitoring delivers value in modern operating rooms
The most transformative shift in intraoperative nerve monitoring is the move toward deeper integration with surgical workflows and adjacent technologies. Monitoring is no longer treated as a separate step managed in isolation; instead, it is increasingly expected to coordinate with anesthesia protocols, patient positioning, and surgical navigation practices. As teams standardize intraoperative checklists and safety processes, monitoring systems that reduce setup time, simplify electrode placement, and provide clear, actionable alerts gain a practical advantage.Another major change is the rise of software-centric differentiation. While signal acquisition remains foundational, the emphasis is shifting toward how effectively systems filter noise, display meaningful trends, and help teams interpret changes in real time. Clinical users increasingly demand interfaces that support rapid comprehension under pressure, especially in high-stakes procedures where false positives can disrupt flow and false negatives can be catastrophic. This has accelerated interest in advanced algorithms, analytics-enabled dashboards, and smarter configuration tools that reduce dependence on highly specialized operator intuition.
Service delivery models are also transforming the landscape. In many settings, hospitals rely on third-party monitoring services or contracted technologists to address staffing constraints and variability in in-house expertise. This trend is influencing vendor strategies around training, remote support, and partnerships. Furthermore, the push toward ambulatory surgery and shorter length-of-stay pathways is creating pressure to deliver high-quality monitoring with smaller OR teams and faster turnover, elevating the importance of reliability, portability, and standardized protocols.
Finally, procurement and risk management stakeholders are becoming more prominent in adoption decisions. Beyond surgeon preference, value analysis committees increasingly scrutinize evidence, total cost, and utilization consistency. This broadening of decision influence is driving suppliers to articulate economic and operational value, not just clinical capability, and to demonstrate how monitoring systems can be implemented at scale without introducing friction into OR operations.
United States tariffs in 2025 are reshaping cost structures and supply chain resilience, influencing pricing, sourcing, and clinical continuity for nerve monitoring
The cumulative impact of United States tariffs in 2025 introduces meaningful considerations for the intraoperative nerve monitoring ecosystem, particularly because many systems and components rely on globally distributed supply chains. Even when final assembly occurs domestically, key inputs such as electronic subassemblies, sensor materials, cables, connectors, and specialized manufacturing equipment may be imported. Tariff-driven cost pressure can therefore appear in unexpected places, affecting not only capital equipment pricing but also the recurring economics of disposables and accessories.For manufacturers, the operational response is likely to include intensified supplier diversification, redesign efforts to qualify alternative components, and selective localization of production for tariff-sensitive elements. However, these changes are not frictionless. Requalification may trigger additional verification activities, updates to quality documentation, and potential lead-time disruptions during transitions. In regulated environments, even seemingly minor component substitutions can require careful validation to maintain performance consistency, which makes rapid changes harder than in less regulated device categories.
For providers, tariffs can translate into tighter procurement negotiations and a greater emphasis on contract structures that stabilize pricing over time. Hospitals may increasingly seek bundled agreements that account for both the base system and ongoing electrode consumption, while also demanding greater transparency on what drives recurring costs. In parallel, supply chain resilience becomes a clinical risk topic: delayed delivery of electrodes or replacement cables can interrupt scheduled cases or force last-minute substitutions that disrupt established protocols.
Strategically, the 2025 tariff environment may also accelerate consolidation and partnership behavior. Larger firms with greater purchasing power, broader supplier networks, and more flexible manufacturing footprints can better absorb volatility than smaller entrants. As a result, competitive advantages may increasingly stem from operational resilience and regulatory-ready sourcing, not only from signal performance. Companies that proactively communicate continuity plans and demonstrate stable availability are positioned to strengthen trust with hospital administrators and OR leadership.
Looking ahead, the most successful organizations will treat tariff exposure as a design and commercial planning variable, not merely a finance issue. By embedding supply-chain risk assessment into product roadmap decisions and by aligning sales promises with realistic fulfillment capabilities, stakeholders can reduce downstream disruption and protect the clinical reliability that nerve monitoring ultimately depends on.
Segmentation patterns reveal how modality mix, system configuration, component economics, and end-user workflow differences shape real-world adoption decisions
Segmentation insights in intraoperative nerve monitoring systems become most actionable when they connect purchasing behavior to clinical workflow realities. Across system type, the contrast between portable and cart-based configurations highlights how site-of-care priorities shape adoption. Portable designs often align with facilities optimizing room turnover and space utilization, whereas cart-based setups can remain favored in complex environments where multiple modalities, larger displays, and robust accessory management support high-acuity cases. This distinction becomes even more pronounced as ambulatory surgery expands and hospitals evaluate how to standardize equipment across multiple sites.From a modality perspective spanning EMG, SSEP, MEP, and EEG, utilization patterns reflect both procedure risk profiles and institutional capability. EMG frequently serves as a foundational modality due to its practicality for many nerve-at-risk procedures, while SSEP and MEP are often emphasized in cases where spinal cord and long-tract integrity are central concerns. EEG plays a more selective role, commonly tied to neurophysiologic assessment requirements in particular clinical contexts. As clinicians become more accustomed to multimodal approaches, vendors that deliver consistent performance across modalities-without adding complexity-can differentiate in competitive evaluations.
The segmentation lens of product components spanning electrodes, probes, stimulators, amplifiers, accessories, and software underscores where ongoing value is created beyond the initial system sale. Electrodes and accessories are tightly linked to recurring utilization, protocol standardization, and supply continuity. Software, meanwhile, is increasingly a strategic differentiator because it influences usability, signal interpretation, reporting quality, and integration with hospital documentation practices. The result is that purchasing decisions are less about a single device transaction and more about an ecosystem choice that affects day-to-day execution.
Insights by technology type across standalone and integrated systems illuminate a broader transformation toward connected operating rooms. Standalone systems may remain viable when departments seek independence, rapid deployment, or simplified maintenance. Integrated approaches, however, can align with institutions investing in interoperability, centralized IT governance, and coordinated capital planning across OR technologies. Integration is also gaining relevance as hospitals aim to standardize data capture, reduce documentation gaps, and support quality initiatives that require consistent intraoperative records.
Finally, segmentation by end user across hospitals, ambulatory surgical centers, and specialty clinics clarifies why a single go-to-market message rarely works. Hospitals often weigh breadth of clinical use, service coverage, and long-term durability. Ambulatory surgical centers prioritize speed, reliability, and compact setups that fit lean staffing models. Specialty clinics may focus on targeted procedural workflows, clinician preference, and cost control with narrower modality needs. Suppliers that tailor training, service, and packaging to these distinct operational contexts can improve adoption consistency and reduce friction after installation.
Regional adoption differs across the Americas, Europe Middle East & Africa, and Asia-Pacific as infrastructure, procurement rigor, and clinical capacity shape demand
Regional dynamics in intraoperative nerve monitoring reflect differences in procedure mix, care delivery models, and regulatory and reimbursement environments. In the Americas, adoption tends to be influenced by a combination of risk management culture, clinical specialization, and procurement governance that increasingly involves value analysis. Large integrated delivery networks can drive standardization across sites, which favors vendors able to support broad training and dependable logistics. At the same time, staffing variability and the availability of neurophysiologic expertise continue to influence whether hospitals lean toward in-house programs or contracted monitoring services.In Europe, Middle East & Africa, the landscape is shaped by heterogeneous healthcare systems and differing levels of surgical infrastructure maturity. In many European markets, strong clinical standards and structured procurement processes can support adoption when evidence and guideline alignment are clear, while budget constraints in certain settings heighten focus on total lifecycle cost and utilization efficiency. Across parts of the Middle East, investment in advanced surgical capabilities and flagship hospital projects can elevate demand for modern monitoring platforms, yet ongoing workforce development and training capacity remain critical to sustained utilization. In several African markets, access considerations, capital allocation constraints, and service coverage can influence the pace and depth of adoption, with opportunities often tied to centers of excellence and partnerships that build clinical capability.
In Asia-Pacific, growth in surgical volumes, expanding private hospital networks, and modernization of operating rooms are important tailwinds for nerve monitoring adoption. However, the region’s diversity means purchasing priorities can range from premium, feature-rich systems in high-resource urban centers to value-optimized configurations in cost-sensitive markets. Local regulatory pathways, import policies, and distribution capabilities can materially affect availability and after-sales support. Additionally, training and standardized protocols play an outsized role as new centers develop neurophysiology programs, creating demand for solutions that simplify setup, reduce operator burden, and offer robust education resources.
Across all regions, a common thread is the increasing importance of reliability and service responsiveness. Facilities are less tolerant of workflow disruption and more attentive to vendor capability in supporting accessories, consumables, and timely technical assistance. As a result, regional strategy must balance product performance with practical execution factors such as logistics, training infrastructure, and the ability to align solutions to local procedural needs and staffing models.
Competitive advantage increasingly depends on combining neurophysiology expertise with dependable service, software innovation, and lifecycle support for OR teams
Company dynamics in intraoperative nerve monitoring are increasingly defined by how effectively suppliers pair clinical credibility with operational execution. Established medical device organizations often benefit from broad distribution, strong service infrastructure, and the ability to bundle offerings across adjacent surgical categories. This can be advantageous in competitive tenders where hospitals prioritize vendor stability, uptime assurances, and standardized training programs across multiple sites.Specialized neurophysiology-focused players, by contrast, may differentiate through deep modality expertise, refined user interfaces, and close alignment with clinical workflows in high-acuity procedures. Their competitive edge often depends on responsiveness to clinician feedback, continued software refinement, and strong educational support that builds confidence among surgeons, anesthesiologists, and technologists. In many cases, their ability to partner with monitoring service providers or to offer remote support frameworks becomes a practical growth lever.
Innovation trajectories are also shaping company positioning. Suppliers investing in improved signal processing, noise reduction, and intuitive alerting systems are responding directly to end-user demands for clarity under time pressure. Meanwhile, enhanced reporting tools and compatibility with documentation workflows are becoming more prominent, as hospitals seek better traceability and more consistent intraoperative records. Companies that treat interoperability and cybersecurity as core product requirements-rather than afterthoughts-are better aligned with hospital IT governance trends.
Commercial strategy increasingly hinges on consumables and service. Electrode portfolios, accessory availability, and predictable replenishment can be as decisive as the base platform, particularly for facilities aiming to standardize protocols. In parallel, training and competency-building offerings are becoming more formalized, with suppliers that provide structured onboarding and continuing education often achieving stronger utilization after installation.
Overall, competitive advantage is shifting toward organizations that can demonstrate both clinical performance and dependable execution across the full lifecycle, from procurement and installation through ongoing case support and supply continuity.
Leaders can win by standardizing workflows, hardening supply resilience, expanding service models, and proving value to both clinicians and administrators
Industry leaders can strengthen positioning by prioritizing solutions that reduce variability in setup and interpretation. Standardizing electrode placement guidance, simplifying system configuration, and improving intraoperative alert clarity can directly address adoption barriers tied to staffing differences and training gaps. In parallel, investing in workflow-aligned user experience design helps ensure monitoring contributes to surgical confidence rather than adding cognitive load.Leaders should also treat supply chain resilience as a core element of product strategy, especially amid tariff-driven volatility. Qualifying alternative suppliers, maintaining dual sourcing for critical components, and building transparent continuity plans can reduce disruption risk for hospitals. Commercial teams can reinforce trust by aligning contract language with realistic lead times and by offering service-level commitments that protect case scheduling.
Partnership and service models deserve equal attention. Where technologist availability is constrained, leaders can expand adoption by supporting remote monitoring capabilities, scalable training programs, and collaboration frameworks with third-party service providers. These approaches should be coupled with clear clinical governance practices so hospitals can maintain consistent standards across teams and sites.
From a product roadmap perspective, industry leaders should focus on software capabilities that materially improve decision-making. This includes robust artifact management, trend visualization tailored to specific procedure types, and reporting tools that streamline documentation. Interoperability planning should be proactive, with secure data handling and compatibility considerations addressed early to meet hospital IT requirements.
Finally, leaders should refine value communication for a broader set of stakeholders. Surgeons may prioritize performance and usability, while administrators and value analysis committees focus on utilization consistency, training burden, and predictable operating costs. A unified narrative that connects clinical outcomes, operational efficiency, and risk management will be more persuasive than feature-centric messaging.
A triangulated methodology blending stakeholder interviews, product and regulatory review, and consistent segmentation logic supports decision-ready market understanding
The research methodology for this report integrates structured primary and secondary approaches to build a grounded, decision-ready view of the intraoperative nerve monitoring system landscape. The work begins with scoping that defines the market boundaries, key technologies, and clinical applications, ensuring terminology and categorization align with how providers and manufacturers describe real-world usage.Primary research incorporates interviews and discussions with stakeholders across the ecosystem, including clinical users involved in neurophysiology and surgical practice, procurement and value analysis participants, and industry representatives responsible for product strategy and commercialization. These conversations are used to validate workflow trends, purchasing drivers, unmet needs, and adoption barriers, with attention paid to differences by site of care and procedure type.
Secondary research synthesizes information from publicly available materials such as regulatory filings, company disclosures, product documentation, clinical and professional association publications, standards and guidance documents, and broader healthcare policy updates. This step helps triangulate claims, confirm technology directions, and contextualize operational factors such as supply chain and compliance expectations.
Data triangulation is applied throughout to reconcile differences across sources and to minimize bias from any single viewpoint. The analysis uses consistent segmentation logic and cross-checks themes across stakeholder groups to ensure insights reflect both clinical realities and commercial constraints. Quality assurance includes editorial review for clarity and internal consistency, with iterative refinement to maintain a coherent narrative that supports executive decision-making.
The nerve monitoring landscape rewards clinically credible, operationally resilient solutions that align technology, service delivery, and procurement realities
Intraoperative nerve monitoring systems are becoming more central to how surgical teams manage risk in nerve-at-risk procedures, and the category is moving toward deeper workflow integration, stronger software-driven differentiation, and more flexible service models. As hospitals balance patient safety priorities with operational constraints, the systems that succeed will be those that deliver dependable signals, intuitive interpretation, and practical implementation support.At the same time, the external environment is adding complexity. Tariff-related cost pressures and supply chain volatility in 2025 elevate the importance of resilient sourcing, predictable availability of consumables, and transparent vendor commitments. These factors increasingly influence purchasing decisions alongside clinical performance.
Segmentation and regional patterns make clear that adoption is not uniform. Needs differ by modality mix, system configuration, end-user setting, and regional infrastructure. Consequently, suppliers and providers benefit from strategies that account for real workflow conditions, training capacity, and procurement governance rather than relying on one-size-fits-all assumptions.
Taken together, the landscape rewards organizations that combine clinical credibility with operational excellence. Those that align technology innovation with service reliability, interoperability expectations, and clear stakeholder value articulation will be best positioned to support safer surgeries and sustainable adoption.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Intraoperative Nerve Monitoring System Market
Companies Mentioned
The key companies profiled in this Intraoperative Nerve Monitoring System market report include:- 3B Scientific GmbH
- A-M Systems, Inc.
- Alpha Omega Engineering
- American Intraoperative Monitoring
- Axon Healthcare
- B. Braun Melsungen AG
- Bovie Medical
- Cadwell Industries
- Checkpoint Surgical
- Compumedics
- Dr. Langer Medical
- Drägerwerk AG & Co. KGaA
- Erbe Elektromedizin
- Inomed Medizintechnik GmbH
- IntraNerve Neuroscience Holdings
- Magstim
- Masimo Corporation
- Medtronic plc
- Moberg ICU Solutions
- Natus Medical
- NeuroMonitoring Technologies
- Neurovision Medical Products
- NeuroWave Systems
- Nihon Kohden Corporation
- NuVasive
- ProPep Surgical
- Sentient Medical Systems
- SpecialtyCare
- Stryker Corporation
- Synapse Biomedical
- Xavant Technology Pty Ltd
- Zimmer Biomet
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
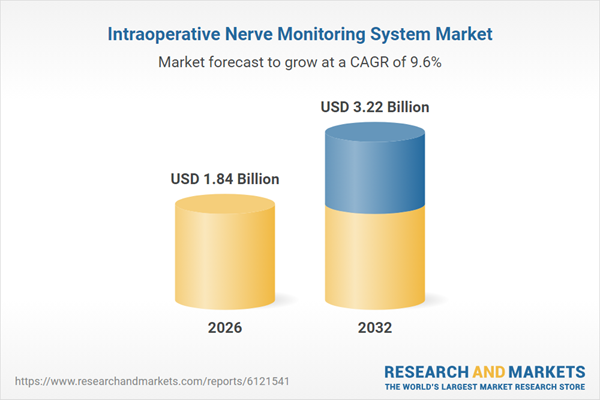
| Estimated Market Value ( USD | $ 1.84 Billion |
| Forecasted Market Value ( USD | $ 3.22 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 33 |