Speak directly to the analyst to clarify any post sales queries you may have.
Absorbable dermal fillers are entering a more evidence-driven and consumer-skeptical era where safety, subtle outcomes, and supply resilience shape growth
Absorbable dermal fillers have become a central tool in modern aesthetic medicine, bridging patient demand for natural-looking enhancement with clinicians’ preference for predictable, reversible, and well-characterized materials. As minimally invasive procedures continue to normalize across age groups and geographies, fillers increasingly function as both a first-line aesthetic intervention and a complement to energy-based devices and surgical approaches. This evolution is reinforced by the growing sophistication of injection techniques, expanded indications beyond simple wrinkle correction, and heightened expectations around safety, longevity, and tissue integration.At the same time, the category is being reshaped by more discerning consumers who prioritize subtlety, personalization, and evidence-backed product claims. Patients increasingly ask about ingredient provenance, duration of effect, adverse event management, and how a given filler behaves in different facial planes. Clinicians, in turn, are adopting more structured treatment planning that balances immediate visual improvement with longer-term tissue support, especially for midface volumization and jawline definition.
Against this backdrop, the competitive environment is intensifying. Established brands are defending share through training ecosystems and portfolio breadth, while newer entrants focus on differentiated rheology, improved crosslinking strategies, and delivery formats that streamline workflow. Regulatory expectations, supply chain resilience, and the economics of practice are now as influential as raw clinical performance. This executive summary frames the shifts, operational implications, segmentation logic, regional dynamics, and strategic actions that matter most for stakeholders operating in absorbable dermal fillers.
From technique evolution to tighter compliance, the filler ecosystem is shifting toward outcomes accountability, clinician education, and fortified distribution controls
The landscape is undergoing transformative shifts that extend well beyond incremental formulation updates. One of the most consequential changes is the move from volume replacement alone to integrated facial architecture planning. Clinicians increasingly treat the face as a dynamic system, considering ligament support, fat compartment behavior, and skin quality, which places a premium on fillers with predictable lift, controlled spread, and tissue compatibility. This has elevated the importance of rheology and handling characteristics, not just duration.In parallel, the market is seeing a clearer separation between premium medically supervised channels and price-pressured offerings that compete on accessibility. While affordability remains important, a growing share of decision-making is anchored in risk management, traceability, and brand-backed complication protocols. As more jurisdictions scrutinize who can inject and under what supervision, companies that can provide robust training, adverse event resources, and compliant promotional practices are gaining strategic advantage.
Technology and data are also reshaping commercial execution. Practices are adopting digital consultation tools, before-and-after imaging, and standardized consent workflows, which raises expectations for manufacturers to provide patient education assets that are accurate and regulator-friendly. Meanwhile, social media influence remains strong, but it is maturing into a more outcomes-oriented conversation, with patients comparing longevity, swelling profiles, and recovery time rather than simply chasing dramatic transformations.
Finally, supply chain strategy has become a differentiator. Greater attention to cold-chain integrity, packaging security, and anti-counterfeiting measures is changing how products are distributed and authenticated. As counterfeit risk persists in parts of the value chain, manufacturers are investing in serialization, tamper-evident packaging, and tighter distributor governance. These shifts collectively reward companies that treat fillers as a clinical platform supported by education, compliance, and reliable fulfillment rather than a standalone product.
United States tariff pressures in 2025 are reshaping sourcing, validation decisions, and channel economics, making resilient operations a competitive advantage
The 2025 tariff environment in the United States introduces a cumulative set of pressures that ripple across pricing, sourcing, and inventory strategy for absorbable dermal fillers. Even when finished fillers are not directly targeted, tariffs affecting upstream inputs such as medical-grade polymers, specialty chemicals, packaging components, and cold-chain logistics materials can increase landed costs and complicate procurement planning. For manufacturers with globalized production footprints, the result is a more complex cost stack that must be managed without undermining quality or regulatory commitments.These tariff dynamics also influence where value is created along the chain. Companies may accelerate localization of select steps, such as secondary packaging, labeling, or final kitting, to reduce exposure and improve responsiveness. However, moving regulated activities is not trivial; it requires revalidation, documentation updates, and sometimes regulatory notifications. Consequently, firms are likely to adopt a hybrid approach that balances stable, validated manufacturing with more flexible downstream operations. Over time, this can shift competitive advantage toward players with strong quality systems and cross-functional coordination between regulatory, operations, and commercial teams.
Downstream, the tariff environment can subtly reshape channel behavior. Distributors and large practice groups may push for longer price locks, higher service levels, or alternative sourcing to protect margin. Clinics facing higher input costs may adjust patient pricing, rebalance product mix, or tighten inventory to avoid expiration risk. In response, manufacturers that offer dependable allocation policies, transparent communication, and practical ordering cadence support can strengthen loyalty even in a cost-sensitive setting.
Importantly, tariffs can amplify the need for robust risk governance. Firms should expect heightened scrutiny of product origin claims, documentation accuracy, and customs compliance. This makes traceability, supplier qualification, and harmonized product documentation central not only to regulatory readiness but also to commercial continuity. In a category where patient safety and brand trust are paramount, operational disruptions tied to trade policy can quickly become reputational challenges if not proactively managed.
Segmentation clarifies how material choice, indication-specific technique, care setting economics, and channel governance jointly determine adoption and loyalty
Segmentation in absorbable dermal fillers reveals how performance expectations and purchasing behavior vary by material science, clinical application, end-user setting, and distribution route. Product differentiation is most visible when considering hyaluronic acid-based fillers compared with calcium hydroxylapatite, poly-L-lactic acid, and polymethylmethacrylate-based options that are used in absorbable or bio-stimulatory contexts. Hyaluronic acid remains closely associated with versatility, reversibility via hyaluronidase, and a broad range of textures suited to superficial lines through deep volumization. By contrast, calcium hydroxylapatite and poly-L-lactic acid are frequently evaluated through the lens of structural support and collagen stimulation, which can align well with full-face rejuvenation philosophies but require different patient counseling and technique discipline.Clinical indications shape portfolio strategy as strongly as material type. Use cases spanning facial line correction, lip augmentation, and midface volumization each carry distinct expectations around swelling, softness, projection, and longevity. Demand for jawline contouring and chin enhancement continues to emphasize products that provide lift and definition while maintaining a natural feel in motion. Meanwhile, the market for under-eye and perioral treatments is increasingly cautious, favoring fillers with controlled hydration and spread to reduce the risk of visible irregularities. This pushes manufacturers to position products with clear plane-of-injection guidance and evidence-based complication mitigation recommendations.
End-user segmentation highlights operational realities that influence adoption. Dermatology clinics and plastic surgery clinics tend to prioritize portfolio breadth, advanced training, and consistent outcomes across diverse patient types. Medical spas, particularly those aligned with physician oversight, often value workflow efficiency, patient experience assets, and dependable replenishment cycles. Hospitals, where they participate in aesthetics, may be more sensitive to procurement protocols, vendor credentialing, and documentation standards. Each setting can require a different mix of clinical support, commercial contracting, and educational programming.
Distribution segmentation adds another layer of strategy. Direct sales models can deepen relationships through training and practice development support, while third-party distributors can expand reach but require tighter governance to protect against diversion and counterfeits. As e-commerce influences patient discovery and appointment conversion, manufacturers also face rising expectations for digital education, authenticity verification tools, and post-treatment guidance that supports adherence and satisfaction. Taken together, segmentation underscores that success depends on aligning material properties and clinical outcomes with the operational needs of each end-user and the risk profile of each channel.
Regional performance hinges on how well brands adapt to regulatory nuance, injector education norms, and culturally specific aesthetic ideals across major geographies
Regional dynamics in absorbable dermal fillers reflect differences in regulatory structures, injector training norms, consumer preferences, and channel maturity across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, demand is shaped by high procedure volumes, sophisticated marketing, and a strong culture of brand-led training. Patients often seek natural refinement and preventative approaches, while practices increasingly bundle fillers with neuromodulators and energy-based devices. Operationally, the region’s emphasis on compliance and litigation awareness makes traceability, adverse event support, and clear labeling central to brand credibility.Europe presents a diverse operating environment where country-specific reimbursement boundaries, advertising rules, and injector qualifications influence how products are positioned and sold. Many markets place strong emphasis on clinician education and conservative aesthetic ideals, which favors products with predictable integration and lower incidence of visible irregularities. At the same time, cross-border movement and parallel trade risks can be more pronounced, elevating the importance of authenticated distribution and consistent product documentation across languages and jurisdictions.
The Middle East & Africa region combines high aesthetic awareness in key urban centers with variability in regulatory maturity and healthcare infrastructure. Premiumization can be pronounced in certain markets, with strong demand for contouring, defined profiles, and high-touch clinic experiences. This creates opportunities for companies that invest in elite injector training, culturally aligned patient education, and reliable cold-chain logistics. However, the region’s heterogeneity requires careful partner selection and disciplined channel oversight.
Asia-Pacific continues to be shaped by rapid adoption of minimally invasive aesthetics, evolving beauty ideals, and expanding networks of clinics. Market behavior often rewards products that support precision, subtle enhancement, and efficient appointment workflows. Differences in facial anatomy considerations and treatment goals can influence preferred filler characteristics, such as cohesivity and projection. As regulatory standards tighten in several countries, manufacturers that can combine strong clinical evidence, localized education, and robust authentication measures are better positioned to scale sustainably.
Across all regions, the balance between innovation and trust is decisive. Companies that treat regional strategy as a blend of clinical training, compliant communication, and resilient fulfillment-rather than simply a sales expansion plan-tend to build more durable positions.
Competitive advantage is increasingly earned through integrated portfolios, training ecosystems, and anti-counterfeit governance as much as through formulation innovation
Company competition in absorbable dermal fillers is increasingly defined by portfolio architecture, education ecosystems, and the ability to operationalize safety at scale. Leading players typically maintain multi-density product lines designed for different facial planes and indications, paired with cohesive clinical guidance that standardizes outcomes across injector skill levels. They also invest heavily in hands-on training, key opinion leader networks, and digital learning platforms that help clinics refine technique and manage complications responsibly.Innovation trajectories are converging around improved rheology control, longer-lasting yet natural-feeling results, and more predictable tissue interaction. Manufacturers differentiate through crosslinking technologies, particle design, and formulation choices that influence lift, spread, and hydration behavior. Just as important is delivery experience: ergonomically designed syringes, consistent extrusion force, and needle or cannula compatibility can meaningfully affect clinician preference and repeat purchasing.
Beyond the product itself, companies are being judged on governance and reliability. Strong anti-counterfeiting measures, lot-level traceability, and disciplined distributor management have become table stakes in markets where diversion can erode trust. In addition, firms that provide clear clinical incident pathways-covering vascular compromise recognition, protocol guidance, and education on reversal where applicable-are better aligned with the industry’s shift toward accountability.
Commercially, strategic partnerships with large clinic groups and integrated aesthetics platforms are becoming more common, especially where bundled purchasing and standardized protocols drive efficiency. Companies that combine scientific credibility with practice growth support-such as patient education materials, consent templates, and outcome documentation tools-are often better positioned to embed their products into repeatable care pathways. In this environment, sustainable leadership is built less on single hero products and more on integrated capabilities that align R&D, medical affairs, quality, and field education.
Leaders can win by pairing supply-chain resilience and tariff-ready sourcing with outcomes-based education, stronger channel control, and clinic enablement tools
Industry leaders can respond to current conditions by prioritizing operational resilience alongside clinical differentiation. Strengthening supplier qualification, dual-sourcing critical inputs, and improving demand planning tied to clinic consumption patterns can reduce the likelihood of allocation events that damage loyalty. In parallel, reviewing tariff exposure across materials, components, and logistics can identify where localized downstream steps or redesigned packaging may reduce risk without triggering avoidable regulatory complexity.On the clinical side, companies should elevate education from product training to outcomes training. That means investing in curricula that address patient selection, plane-of-injection decision-making, combination therapy sequencing, and complication preparedness. Because adverse events can become highly visible, a structured approach to incident support and clinician communication helps protect both patients and brands. Where hyaluronic acid products are marketed, responsible guidance on reversal and management protocols should be clear, practical, and aligned with local regulations.
Commercial strategy should also reflect shifting patient expectations. Support clinics with compliant patient education that explains what “absorbable” means, sets realistic timelines, and reinforces the importance of qualified injectors. Moreover, enabling clinics to document outcomes consistently-through photography standards, follow-up cadence, and satisfaction measures-can strengthen repeat treatment pathways while generating real-world insights for medical affairs.
Finally, treat channel governance as a growth lever rather than a constraint. Tightening distributor authorization, implementing robust track-and-trace practices, and using authentication tools that clinics can easily verify helps reduce diversion and counterfeit exposure. Over time, these steps support premium positioning, reduce medical risk, and create a more defensible commercial footprint even as competitive intensity rises.
A triangulated methodology combining regulatory review, clinical signals, and stakeholder validation connects product science to real-world purchasing and practice behavior
The research methodology for this report integrates structured secondary review with primary engagement to capture both technical realities and commercial behavior in absorbable dermal fillers. The secondary phase consolidates publicly available regulatory guidance, product documentation, clinical literature, patent and technology signals, and corporate communications to map how the category is evolving in materials, indications, and safety expectations. This provides a disciplined baseline for understanding what is clinically plausible, what is regulatorily permitted, and where innovation is concentrated.Primary inputs are then used to validate assumptions and reveal practitioner-level decision drivers. Interviews and discussions with stakeholders such as clinicians, clinic operators, distributors, and industry experts help clarify how products are selected, what training gaps exist, and how purchasing patterns respond to pricing, service levels, and supply consistency. These insights are particularly important in a procedure-driven category where preferences can shift based on handling feel, complication support, and patient satisfaction, not just published claims.
Triangulation is applied throughout to reduce bias and ensure consistency. Conflicting signals-such as differences between marketing narratives and clinical practice realities-are resolved through follow-up validation and cross-comparison across stakeholder groups and regions. The analysis also evaluates channel integrity considerations, including authentication practices and distribution structures, because these directly affect brand trust and patient safety.
Finally, the methodology emphasizes relevance to decision-makers. Findings are organized to connect product attributes to clinical use cases, end-user economics, and regional operating constraints. This approach helps stakeholders translate complex technical and commercial signals into practical strategic choices.
As the category matures, durable success will come from predictable outcomes, disciplined safety systems, and region-ready execution that protects trust end to end
Absorbable dermal fillers are no longer competing primarily on availability or basic efficacy; they are competing on predictability, safety governance, and the ability to support clinicians in delivering consistent, natural outcomes. As technique standards mature and patients become more informed, the winners will be those who align material science with transparent education, compliant communication, and robust complication readiness.At the same time, operational variables-from tariff-related cost pressures to distribution integrity-are now strategic factors rather than back-office concerns. Companies that anticipate friction in sourcing and logistics, and that protect channel authenticity, can preserve trust and continuity even in volatile conditions.
Ultimately, the category’s direction points toward integrated platforms: diversified portfolios tuned to specific indications, strong training ecosystems, and regionally adapted execution. Stakeholders who act on these priorities will be better positioned to build durable relationships with clinics and deliver patient experiences that sustain demand over time.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Absorbable Dermal Fillers Market
Companies Mentioned
The key companies profiled in this Absorbable Dermal Fillers market report include:- AbbVie Inc.
- Anika Therapeutics, Inc.
- Beijing IMEIK Technology Development Co., Ltd.
- Bioha Laboratories
- BioPlus Co., Ltd.
- Bioxis Pharmaceuticals
- Bloomage BioTechnology
- Croma‑Pharma GmbH
- Dr. Korman Laboratories
- Galderma S.A.
- Haohai Biological Technology
- Hugel, Inc.
- Huons BioPharma
- Jingjia Medical Technology
- LG Chem Ltd.
- Medytox
- Merz Pharma GmbH & Co. KGaA
- Prollenium Medical Technologies, Inc.
- Revance Therapeutics, Inc.
- SciVision Biotech
- Sinclair Pharma Limited
- Suneva Medical, Inc.
- Teoxane SA
- Zimmer Aesthetics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
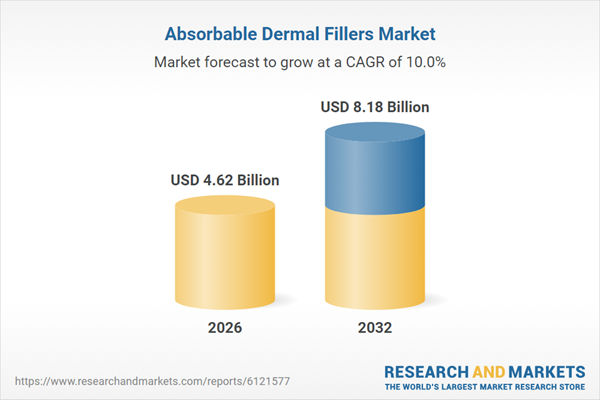
| Estimated Market Value ( USD | $ 4.62 Billion |
| Forecasted Market Value ( USD | $ 8.18 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |