Speak directly to the analyst to clarify any post sales queries you may have.
Setting the context for PCV13 as immunization policies evolve, serotype dynamics shift, and program decisions demand sharper operational focus
Pneumococcal disease remains a persistent cause of morbidity and mortality, particularly among infants, older adults, and individuals with certain chronic or immunocompromising conditions. The pneumococcal 13-valent conjugate vaccine (PCV13) has been a pivotal intervention because conjugate technology is designed to generate durable, T-cell-dependent immune responses and reduce carriage for vaccine-included serotypes, which in turn supports broader community protection.However, the strategic context for PCV13 is not static. Over time, serotype distribution has shifted in response to vaccination programs, and national immunization advisory groups have adapted guidance to reflect evolving epidemiology, the availability of newer higher-valent conjugate vaccines, and real-world program outcomes. As a result, decisions around PCV13 are increasingly about fit-for-purpose use cases, access economics, and operational continuity rather than one-size-fits-all deployment.
This executive summary frames the market environment for PCV13 through the lens of what matters most to decision-makers: changing clinical and policy priorities, supply and trade dynamics, the segmentation logic that determines adoption pathways, regional implementation realities, and the competitive behaviors shaping procurement and partnerships. It also emphasizes actions that can strengthen resilience and support appropriate vaccine use within immunization systems that must balance affordability, coverage, and confidence.
How higher-valent competition, evidence-driven policy updates, and post-pandemic resilience priorities are reshaping PCV13 decision-making
The PCV13 landscape has entered a phase defined less by initial adoption and more by optimization amid expanding options. A central shift is the movement from broad pediatric program scale-up toward more differentiated strategies that weigh local serotype prevalence, catch-up needs, and the role of higher-valent conjugate vaccines in both pediatric and adult populations. In several settings, guidance has increasingly favored tailored adult vaccination decisions through shared clinical decision-making rather than universal age-based approaches, reflecting assessments of incremental benefit in populations already indirectly protected through pediatric immunization.At the same time, stakeholders have elevated “program performance” metrics beyond coverage rates. Health systems are paying closer attention to schedule adherence, missed-opportunity reduction, cold-chain reliability, and end-to-end data traceability. This is partly driven by post-pandemic operational lessons: immunization programs must withstand workforce disruptions, clinic backlogs, and supply fluctuations without eroding public trust.
Another transformative shift is the acceleration of lifecycle management and portfolio positioning. Manufacturers and procurers are recalibrating how they evaluate PCV13 relative to newer higher-valent alternatives, considering interchangeability policies, tender specifications, and evidence thresholds for switching. This includes more rigorous comparisons of immunogenicity breadth, clinical relevance of additional serotypes, and expected impact on carriage.
Finally, the market has become more “policy-coupled,” where access decisions are tightly linked to national advisory recommendations, reimbursement design, and tender mechanics. Consequently, commercial success increasingly depends on capabilities that sit outside traditional sales execution, such as evidence generation aligned to guideline questions, real-world performance monitoring, supply assurance commitments, and stakeholder education to sustain confidence amid changing recommendations.
Why prospective US tariff pressures in 2025 could ripple through vaccine inputs, cold-chain infrastructure, and contracting behavior across PCV13 supply
United States tariff actions anticipated for 2025 introduce an additional layer of operational and financial complexity for vaccine-adjacent supply chains, even when finished vaccines themselves are protected by public health considerations and procurement norms. The most immediate effect is likely to be felt through inputs and services that surround vaccine production and distribution, including specialized packaging components, cold-chain equipment, laboratory consumables used in quality testing, and certain categories of manufacturing hardware sourced globally.In practical terms, tariffs can create cost variability and lead-time uncertainty, pushing manufacturers and partners to revisit supplier qualification strategies. This encourages dual-sourcing, geographic diversification of suppliers, and higher safety stocks for critical materials, all of which can influence working capital and batch release planning. When such frictions coincide with fixed-price contracting or multi-year public procurement agreements, parties may face tighter margins or the need to renegotiate terms to keep supply stable.
Downstream, the cumulative impact can alter contracting behavior. Purchasers may place greater value on vendors that can demonstrate end-to-end supply risk management, transparent contingency planning, and domestic or tariff-insulated sourcing for key components. This can also reshape distribution strategies, as logistics providers and wholesalers adapt to higher equipment costs and potential bottlenecks in temperature-controlled infrastructure upgrades.
Strategically, the tariff environment reinforces the importance of resilience as a differentiator. Companies that treat trade policy as a scenario-planning input-rather than a one-time cost shock-will be better positioned to maintain continuity of supply and protect immunization program integrity. For PCV13 stakeholders, the message is clear: procurement decisions will increasingly weigh not only price and evidence, but also demonstrable robustness against geopolitical and trade-induced volatility.
Segmentation signals that PCV13 adoption hinges on age, risk, channel, and care setting differences that shape eligibility, workflow, and purchasing logic
Segmentation clarifies how PCV13 demand is shaped by clinical use cases, purchasing pathways, and operational settings, and it is most useful when interpreted as a set of decision filters rather than static categories. When viewed by age group, pediatric indications remain closely tied to national schedule design, including primary series timing and booster practices, whereas adult use is more sensitive to risk stratification, comorbidity prevalence, and provider interpretation of evolving recommendations. This difference matters because pediatric demand tends to be programmatic and forecastable, while adult demand is often influenced by clinical workflow, reimbursement clarity, and patient awareness.When interpreted through indication and risk profile, the highest value for PCV13 frequently concentrates in individuals with immunocompromising conditions, cochlear implants, cerebrospinal fluid leaks, and other medical circumstances that elevate pneumococcal risk. In these segments, clinical protocols and specialist pathways can either accelerate uptake through standardized order sets or suppress uptake when responsibilities are fragmented across care teams. Consequently, companies and health systems that integrate vaccination into specialty care pathways-rather than relying solely on primary care prompts-tend to reduce missed opportunities.
Distribution channel segmentation highlights another set of practical realities. Hospital and integrated delivery network procurement often prioritizes supply assurance, formulary alignment, and predictable contracting, while retail pharmacy administration depends on accessible reimbursement, trained immunizers, and consumer-facing demand generation. Public-sector purchasing introduces additional complexity because tender specifications may define product eligibility, interchangeability expectations, and delivery performance metrics. These channel distinctions influence not only how product moves, but also which stakeholders must be educated and which evidence packages resonate.
Finally, segmentation by end-user setting underscores operational constraints. Routine pediatric visits, adult primary care, specialist clinics, and community pharmacy all have different appointment cadence, documentation systems, and consent workflows. Improving PCV13 performance therefore requires aligning product strategy with the realities of administration environments, including inventory turns, cold-chain capacity, and interoperability of immunization records. Across these segments, the most consistent driver of success is reducing friction: fewer steps to identify eligible patients, fewer barriers at the point of care, and clearer coverage pathways that support confident recommendations.
Regional realities show PCV13 outcomes are shaped by policy maturity, tendering, funding stability, and cold-chain capacity across major geographies
Regional dynamics for PCV13 are best understood as the interaction of policy maturity, funding mechanisms, and health system capacity, which together determine whether vaccination is delivered as a consistent program or as a patchwork of access points. In the Americas, established immunization infrastructures and strong pharmacy footprints can support broad access, yet shifting adult guidance and payer variability make demand more sensitive to reimbursement design and provider education. The region also places high expectations on supply reliability and post-market evidence, pushing stakeholders to maintain clear value narratives amid higher-valent competition.Across Europe, procurement is often strongly influenced by national tendering, health technology assessment perspectives, and country-specific immunization technical advisory group decisions. This can produce rapid changes in product preference when guidance updates occur, and it elevates the importance of local evidence alignment, pharmacoeconomic framing, and stakeholder engagement at the national level. In parallel, differences in adult vaccination uptake across countries make implementation support and clinician-focused communication critical levers.
The Middle East & Africa region presents a spectrum of realities, from countries with mature national immunization programs to settings where coverage is constrained by financing, cold-chain reach, and periodic supply interruptions. Here, operational execution-forecast accuracy, last-mile logistics, and integration with maternal and child health services-often determines real-world impact. Partnerships that strengthen immunization delivery and data quality can be as important as product attributes in sustaining PCV13 access.
In Asia-Pacific, rapid urbanization, diverse payer models, and varying levels of public funding create heterogeneous adoption pathways. Some markets combine robust pediatric coverage with growing adult prevention interest, while others rely more heavily on private purchase and out-of-pocket payment that can limit uptake. This diversity means that stakeholder strategies must be localized: evidence packages should reflect local serotype considerations and clinical practice, and distribution must account for the balance between hospital-based administration and expanding pharmacy or community channels.
Across all regions, a common theme is the rising importance of immunization registries, digital documentation, and catch-up initiatives to address gaps created by service disruptions. As these capabilities mature, they will increasingly shape how PCV13 is positioned, tracked, and evaluated in real-world programs.
Competitive positioning in PCV13 is increasingly defined by evidence credibility, tender execution strength, and supply resilience under higher-valent pressure
Company strategies in the PCV13 arena increasingly reflect a dual imperative: defend clinical relevance while proving operational dependability. Leading participants differentiate through the strength of their evidence base, the clarity of their labeling and guidance-aligned messaging, and the ability to support immunization programs with reliable supply and predictable delivery performance. As higher-valent conjugate vaccines expand options, companies emphasize comparative value narratives that address serotype coverage considerations, real-world effectiveness expectations, and the practicalities of switching within established schedules.Another key differentiator is stakeholder enablement. Organizations that invest in clinician education, patient-facing information that supports vaccine confidence, and tools that streamline eligibility identification can reduce friction at the point of care. This is particularly important in adult segments where demand is more elastic and dependent on provider recommendation strength. In pediatric segments, support for program execution-such as forecasting collaboration, cold-chain guidance, and co-development of implementation materials-can meaningfully influence continuity.
Companies are also strengthening their approach to public procurement and tender competitiveness. Success in tender-driven environments depends not only on pricing discipline, but also on demonstrating consistent fill rates, transparent quality systems, and the capacity to handle surge demand during catch-up campaigns. Additionally, manufacturing resilience and supplier management have become more visible evaluation criteria, especially as governments prioritize health security and continuity of essential medicines.
Finally, competitive advantage is increasingly connected to real-world data capabilities. Firms that can support or collaborate on surveillance, carriage studies, and outcomes monitoring-while respecting privacy and regulatory requirements-are better positioned to respond to policy questions as serotype patterns evolve. In this environment, “proof” is not only clinical trial data but also the ability to provide decision-grade evidence that answers payer, guideline, and programmatic concerns in a timely manner.
What industry leaders can do now to protect continuity, reduce adult pathway friction, and sustain PCV13 relevance amid policy and trade uncertainty
Industry leaders can strengthen PCV13 performance by treating policy evolution as a continuous operating condition rather than an occasional disruption. Align medical, market access, and commercial teams around a single “guideline readiness” plan that anticipates advisory group questions, clarifies the role of PCV13 in current schedules, and provides implementation tools that reduce confusion at the point of care. This includes creating clear guidance for providers on appropriate adult use cases where shared clinical decision-making is relevant.Next, invest in supply chain risk management with the same rigor applied to clinical and regulatory work. Map critical inputs, qualify alternates where feasible, and build contingency plans for packaging, cold-chain materials, and quality testing consumables that could be exposed to trade policy volatility. Where possible, structure contracts to include service-level commitments and transparent risk-sharing mechanisms that protect immunization continuity.
To improve uptake in fragmented adult pathways, embed vaccination into care workflows. Partner with health systems to integrate prompts into electronic health records, standardize standing orders in primary care and specialty clinics, and coordinate with pharmacy networks to capture eligible adults during routine visits. The goal is to transform vaccination from an “extra step” into a default component of chronic disease and preventive care.
Finally, elevate real-world evidence and surveillance collaboration. Support studies and data-sharing frameworks that help stakeholders understand shifting serotype patterns and program outcomes, and ensure that evidence is translated into practical decision aids for payers and immunization managers. By combining operational excellence with credible, localized evidence, leaders can maintain trust and relevance for PCV13 even as the competitive set expands.
Methodology grounded in triangulated evidence, stakeholder validation, and policy-aligned review to reflect real-world PCV13 decision drivers
The research methodology integrates structured secondary research with primary validation to ensure that findings reflect both the scientific foundation of pneumococcal vaccination and the operational realities of procurement and delivery. Secondary research begins with a review of peer-reviewed literature on pneumococcal epidemiology, conjugate vaccine immunology, and serotype dynamics, alongside analysis of publicly available policy statements, immunization schedules, regulatory updates, and tender or procurement frameworks where accessible.Primary research focuses on interviews and consultations with stakeholders who influence or execute PCV13 decisions, including clinicians involved in pediatric and adult immunization, pharmacy and vaccination service leaders, supply chain and procurement professionals, and market access or policy experts. These discussions are used to validate assumptions, clarify workflow barriers, and identify the decision criteria most likely to shape adoption in different settings.
Triangulation is applied across evidence streams to reduce bias and improve reliability. Divergent viewpoints are reconciled by mapping them to context, such as differences in reimbursement models, clinical practice norms, or procurement structures. The research also applies consistency checks to ensure that statements about guidance, clinical use, and operational constraints reflect current realities and do not rely on outdated assumptions.
Finally, insights are synthesized into an executive narrative designed for action. The focus is on decision drivers, risk pathways, and practical implications rather than speculative claims. This approach supports strategic planning, stakeholder alignment, and implementation design for organizations operating within the PCV13 ecosystem.
Closing perspective on PCV13: sustained value depends on evidence-aligned positioning, localized execution, and resilient supply under evolving options
PCV13 remains an important tool within pneumococcal prevention, but the environment around it is increasingly shaped by nuanced policy decisions, evolving serotype considerations, and the expansion of higher-valent alternatives. This has shifted the emphasis from simple adoption to disciplined portfolio positioning, implementation quality, and operational resilience. Organizations that understand how guidance changes translate into workflow behavior will be better equipped to sustain appropriate use.Trade and supply considerations, including tariff-driven uncertainty, reinforce that vaccine strategy cannot be separated from supply continuity and contracting design. Meanwhile, segmentation and regional differences show that a single global playbook is rarely sufficient; success depends on aligning evidence, access, and delivery models to local realities.
Ultimately, the strongest PCV13 strategies combine credible evidence narratives with execution excellence. When stakeholders reduce friction at the point of care, strengthen data visibility, and build resilient supply plans, they create conditions where pneumococcal vaccination programs can remain stable, trusted, and responsive to changing needs.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
15. China Pneumococcal 13-valent Conjugate Vaccine Market
Companies Mentioned
The key companies profiled in this Pneumococcal 13-valent Conjugate Vaccine market report include:- Biological E. Limited
- Chongqing Taibang Biological Products Co., Ltd.
- GlaxoSmithKline plc
- Inventprise
- Merck & Co., Inc.
- Panacea Biotec Ltd.
- Pfizer Inc.
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- Shenzhen Kangtai Biological Products Co., Ltd.
- SK bioscience Co., Ltd.
- Vaxcyte
- Walvax Biotechnology Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
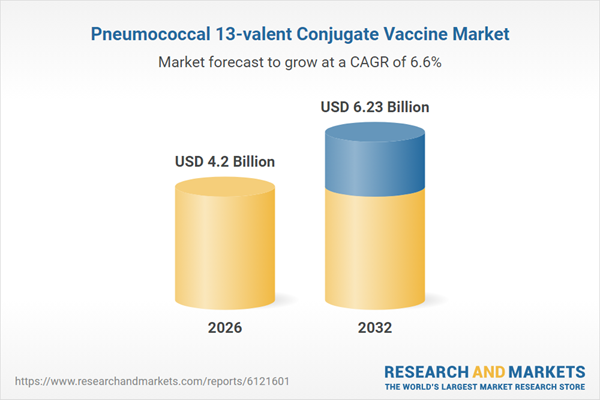
| Estimated Market Value ( USD | $ 4.2 Billion |
| Forecasted Market Value ( USD | $ 6.23 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |