Speak directly to the analyst to clarify any post sales queries you may have.
Tapentadol tablets in modern pain care: why dual‑mechanism analgesia now faces intensified scrutiny, operational complexity, and new supply imperatives
Tapentadol tablets occupy a distinct position in pain management because the molecule combines μ-opioid receptor agonism with norepinephrine reuptake inhibition, offering an analgesic approach that differs mechanistically from many conventional opioids. In clinical practice, this dual action has supported use in moderate-to-severe pain where clinicians are balancing the need for meaningful relief against the imperative to reduce opioid-related harms. As a result, tapentadol sits at the intersection of two powerful forces: sustained demand for effective analgesia and intensifying scrutiny over prescribing, dispensing, and monitoring.Across the healthcare ecosystem, decision-makers are re-evaluating how branded and generic opioid therapies fit into modern care pathways. Formularies, prior authorization rules, and step-therapy requirements increasingly reflect not only clinical evidence and safety profiles but also the operational burden of compliance and the reputational risk associated with misuse and diversion. In parallel, manufacturers and distributors face heightened expectations for transparency, serialization, pharmacovigilance, and supply continuity, especially as regulators and health systems adopt more data-driven oversight.
Against this backdrop, tapentadol tablets are being assessed through a broader lens than efficacy alone. Stakeholders are weighing product differentiation between immediate-release and extended-release options, the practicality of dosing and adherence support, the role of abuse-deterrence narratives, and the resilience of supply chains for controlled substances. This executive summary synthesizes the pivotal shifts reshaping the landscape, the ramifications of U.S. tariff policy in 2025, and the strategic implications across segmentation, regions, and leading companies-culminating in actions industry leaders can take to compete responsibly and sustainably.
Transformative shifts reshaping tapentadol tablets: tighter opioid stewardship, payer friction, compliance-first distribution, and resilience-led manufacturing priorities
The tapentadol tablets landscape is being transformed by a more stringent, systems-level approach to opioid stewardship. Healthcare providers are moving from individual prescriber discretion toward standardized protocols anchored in risk screening, treatment agreements, urine drug testing, and routine checks of prescription drug monitoring programs. Consequently, products that integrate smoothly into these workflows-through clear labeling, practical dosing, and robust safety communication-are better positioned than those that increase administrative friction.At the same time, payer and pharmacy benefit manager practices continue to recalibrate access. Utilization management is increasingly nuanced, with controls varying by indication, dose thresholds, and patient history. This has elevated the importance of evidence packages that speak to real-world outcomes, tolerability, and discontinuation patterns, not simply controlled trial endpoints. In addition, “opioid-sparing” initiatives and multimodal pain strategies have expanded the role of non-opioid alternatives, compelling tapentadol stakeholders to articulate where it provides unique value in carefully selected populations.
Regulatory oversight has also evolved from a primary focus on post-market surveillance to an integrated emphasis on supply-chain accountability. Controlled substance distribution is being monitored for anomalous ordering patterns, and stakeholders are expected to implement sophisticated suspicious order monitoring programs. This shift affects how tapentadol tablets move through wholesale channels, how inventory is allocated, and how distributors manage thresholds and reporting obligations. As a result, reliability of supply is now tightly linked to compliance maturity.
In manufacturing and sourcing, resilience has become as strategic as cost. Companies are diversifying active pharmaceutical ingredient sources, hardening quality systems, and investing in redundancy to mitigate disruptions. Broader geopolitical uncertainty and periodic logistics shocks have reinforced the need for contingency planning, especially for temperature-sensitive shipments, controlled-substance security, and chain-of-custody integrity. Taken together, these changes are reframing competition: success depends not only on product characteristics but on the ability to operate credibly within a more restrictive, data-intensive, and risk-aware ecosystem.
Cumulative impact of 2025 U.S. tariffs: upstream cost shocks, constrained supplier agility for controlled substances, and renewed urgency for nearshoring resilience
United States tariff dynamics in 2025 introduce a new layer of complexity for tapentadol tablet supply chains, even when the finished dosage form is produced domestically. Tariffs can influence the cost and availability of upstream inputs such as active pharmaceutical ingredients, key starting materials, excipients, packaging components, and certain equipment parts. For controlled substances, where supplier qualification and regulatory change control can be time-consuming, sudden cost shocks may be harder to absorb through rapid source switching.As tariff exposure rises, procurement and operations teams are increasingly compelled to map country-of-origin dependencies with greater precision. This includes evaluating multi-tier supplier networks, verifying documentation consistency, and modeling how cost increases flow through contract manufacturing, third-party logistics, and wholesaler pricing structures. In practice, these pressures often trigger renegotiation of long-term supply agreements, rebalancing of safety stock policies, and renewed attention to dual sourcing for critical materials.
Tariffs may also amplify the strategic value of nearshoring and “friendly” sourcing, particularly for firms seeking to reduce geopolitical risk. However, relocating production or qualifying alternative suppliers in the context of controlled substances is not a simple footprint decision. It can require updated regulatory filings, re-validation of processes, stability data bridging, and strengthened security controls. Therefore, the cumulative impact is not merely incremental cost; it is a potential constraint on agility, affecting launch timelines, customer service levels, and the ability to respond to demand variability.
Finally, tariff-driven inflationary pressure can interact with payer cost containment, widening the gap between manufacturing economics and reimbursed net pricing. This increases the importance of operational excellence-yield improvement, batch right-first-time performance, and packaging line efficiency-along with a disciplined contracting strategy that aligns channel partners and limits unplanned margin erosion. Organizations that treat tariffs as a strategic risk domain rather than a procurement annoyance will be better prepared to protect continuity, compliance, and competitiveness.
Key segmentation insights that clarify where tapentadol tablets win: formulation intent, strength-driven access rules, channel friction, and end-user workflow realities
Segmentation dynamics in tapentadol tablets are best understood by examining how use cases and decision criteria vary across product type, strength, distribution channel, and end-user setting, as outlined in the segmentation list. Immediate-release formulations are commonly evaluated for acute pain episodes and breakthrough needs, where speed of onset, titration flexibility, and short-duration prescribing expectations shape selection. Extended-release formulations, by contrast, are judged through the lens of chronic pain management, where stable plasma levels, adherence practicality, and careful patient selection to minimize long-term risks are central to adoption.Strength-based differentiation tends to reflect both clinical titration pathways and payer controls. Lower strengths often align with initiation, cautious escalation, or patients with heightened sensitivity, while higher strengths may be associated with opioid-tolerant populations or complex pain scenarios under specialist supervision. As utilization management becomes more granular, stakeholders increasingly consider how strength availability influences step edits, refill behavior, and the operational workload for prescribers and pharmacies.
Distribution channel segmentation highlights a widening divergence between retail pharmacy workflows and institutional procurement realities. Retail channels are shaped by prior authorizations, pharmacist gatekeeping, and patient affordability considerations, while hospital and clinic settings emphasize formulary positioning, medication safety committee preferences, and protocols for discharge prescribing. In addition, e-pharmacy and mail-order models are being shaped by controlled-substance verification requirements, shipment security standards, and state-by-state dispensing constraints. These channel-specific frictions can materially change the patient journey and, therefore, brand or product preference.
End-user segmentation further clarifies demand signals. Pain clinics and specialty practices often prioritize nuanced titration and monitoring capabilities, whereas general practice settings may be more influenced by standardized guidelines and payer rules that discourage long-term opioid exposure. Meanwhile, inpatient settings focus on continuity of care from acute management to outpatient transition, emphasizing education, tapering plans, and coordination with community pharmacies. Understanding how these segments interact is essential, because the most defensible commercial strategies are those that align product attributes, compliance support, and contracting approaches to the realities of each care setting rather than relying on one-size-fits-all positioning.
Key regional insights across the Americas, Europe, Middle East & Africa, and Asia-Pacific: policy variability, reimbursement structures, and access-defining supply reliability
Regional performance and strategic priorities for tapentadol tablets diverge materially across the regions listed, largely because opioid policy, reimbursement design, and healthcare delivery structures are not uniform. In the Americas, prescribing norms and litigation-era compliance expectations have accelerated adoption of standardized stewardship tools, creating a competitive environment where access is influenced as much by payer rules and monitoring requirements as by clinical preference. This intensifies the need for reliable distribution practices, clear educational materials, and careful alignment with local regulatory expectations.Across Europe, the interplay between national health systems, centralized procurement, and country-specific opioid guidance drives heterogeneous access conditions. Some markets emphasize conservative opioid initiation and structured follow-up, which can influence how immediate-release versus extended-release options are incorporated into care pathways. Additionally, parallel trade considerations, pharmacovigilance rigor, and tendering dynamics can affect availability and continuity, raising the value of strong quality reputation and dependable manufacturing.
In the Middle East & Africa, access is shaped by varying degrees of infrastructure maturity, regulatory harmonization, and controlled-substance import controls. In certain countries, tender-based procurement and limited specialist availability can create episodic demand patterns, while in others, private-sector growth supports more consistent outpatient utilization. Because distribution security and documentation requirements may be stringent, stakeholders often benefit from investing in local partner capability, training, and compliance alignment.
Asia-Pacific presents a broad spectrum of opioid policy restrictiveness, from highly controlled environments to markets expanding pain management capabilities alongside aging populations and rising surgical volumes. Local manufacturing priorities, import dependency, and evolving reimbursement frameworks can significantly affect how tapentadol tablets compete against alternative analgesics. Therefore, regional strategy frequently hinges on navigating regulatory expectations, building credible medical education, and ensuring supply continuity that matches the region’s logistical realities and channel structures.
Key company insights in tapentadol tablets: competitive advantage now hinges on supply assurance, controlled-substance governance, and compliant stakeholder engagement
Competition among key companies in tapentadol tablets is increasingly defined by operational credibility and stakeholder trust rather than promotional intensity. Manufacturers that demonstrate consistent quality performance, strong controlled-substance governance, and rapid responsiveness to audits and inquiries are advantaged in an environment where wholesalers, health systems, and regulators closely scrutinize ordering patterns and distribution practices.Branded positioning continues to matter, particularly where clinical stakeholders perceive differences in evidence depth, educational support, and patient-assistance infrastructure. However, as generic availability expands and procurement becomes more price-sensitive, differentiation often shifts toward supply assurance, contract flexibility, and the ability to support complex channel requirements without triggering compliance concerns. For generics, reliability of raw material sourcing, robustness of quality systems, and continuity planning are pivotal to sustaining long-term relationships with distributors and large pharmacy groups.
Partnership models are also evolving. Contract manufacturing organizations and specialized logistics providers play a larger role in maintaining chain-of-custody integrity, meeting serialization requirements, and executing secure shipments. Companies that invest in collaborative forecasting with channel partners, integrate data signals for anomaly detection, and maintain disciplined inventory governance can reduce stockouts and avoid the reputational risk of irregular distribution patterns.
Finally, companies are increasingly expected to contribute to responsible use through balanced medical education and clear risk communication. In practice, this means ensuring that field teams, medical affairs, and customer support align on compliant messaging, and that stakeholder engagement emphasizes appropriate patient selection and monitoring. Those who combine high compliance maturity with pragmatic customer service are best positioned to sustain access while meeting the heightened expectations surrounding opioid products.
Actionable recommendations for industry leaders: embed stewardship into strategy, tariff-proof supply chains, tailor channel execution, and operationalize compliance excellence
Industry leaders can strengthen position in tapentadol tablets by treating opioid stewardship alignment as a product strategy, not an external constraint. This begins with designing customer-facing resources that reduce friction in prescribing and dispensing workflows, such as clear dosing guidance, conservative titration education, and support for monitoring expectations. When these tools are built for real-world clinic operations, they can improve continuity of care while reinforcing responsible use.Next, organizations should build a tariff- and geopolitics-aware supply strategy that goes beyond supplier price negotiations. Mapping multi-tier supplier dependencies, qualifying alternates for high-risk inputs, and creating playbooks for rapid change control can meaningfully reduce vulnerability. In parallel, investing in manufacturing robustness-deviation reduction, yield stability, and packaging line resilience-helps offset cost pressures without compromising quality.
Commercial strategy should also be tuned to channel-specific realities. Retail access can be improved through proactive payer engagement centered on appropriate-use criteria and evidence communication, while institutional channels benefit from stronger formulary dossiers, medication safety alignment, and discharge pathway education. For mail-order and digital channels, tightening controlled-substance verification, shipment security, and patient communication protocols can prevent operational disruptions and protect trust.
Finally, leaders should operationalize compliance as a measurable capability. Establishing mature suspicious order monitoring, integrating distributor data signals, and training cross-functional teams to respond consistently can reduce regulatory risk. When combined with transparent pharmacovigilance and a culture of quality, these actions support sustainable participation in a category where reputational resilience is inseparable from commercial performance.
Research methodology built for decision readiness: triangulated primary insights, policy and channel analysis, and controlled-substance operational validation
The research methodology underpinning this report integrates structured secondary research, primary expert engagement, and rigorous triangulation to ensure a decision-ready view of the tapentadol tablets environment. Secondary research includes analysis of regulatory and policy publications, controlled-substance compliance frameworks, public manufacturer and distributor information, clinical guideline updates, and documented channel practices affecting opioid access. This foundation is used to map the ecosystem, establish consistent definitions, and identify the most decision-relevant themes.Primary research is conducted through interviews and consultations with stakeholders across the value chain, including pharmaceutical executives, supply-chain and quality leaders, distributors, pharmacists, clinicians involved in pain management, and payer or formulary-adjacent experts where feasible. These conversations are designed to validate real-world workflow constraints, uncover emerging risks, and clarify how procurement and access decisions are being made in practice.
Analytical validation relies on triangulating insights across multiple independent inputs, stress-testing assumptions for internal consistency, and separating confirmed signals from anecdotal viewpoints. Special attention is given to controlled-substance considerations, such as chain-of-custody requirements, suspicious order monitoring expectations, and the operational impact of payer utilization management, because these elements can reshape market behavior even when clinical needs remain steady.
Finally, the output is organized to support action. Segmentation and regional analyses are structured to reveal where access friction concentrates, how supply resilience influences outcomes, and what strategic levers are most practical for manufacturers and partners. This methodology prioritizes transparency, repeatability, and relevance, enabling decision-makers to use the findings confidently in planning, risk management, and execution.
Conclusion: tapentadol tablets demand an integrated strategy where clinical value, access governance, and resilient controlled-substance supply determine success
Tapentadol tablets continue to be evaluated within a pain management environment that demands both therapeutic effectiveness and demonstrable responsibility. The category’s trajectory is influenced by tighter prescribing governance, payer-driven access constraints, and rising expectations for distribution transparency and controlled-substance compliance. These forces are not temporary; they represent a structural evolution in how opioid therapies are selected, monitored, and supplied.At the same time, 2025 tariff pressures underscore that supply-chain strategy is now a core determinant of competitiveness. Cost volatility in upstream inputs, the complexity of qualifying alternate sources, and the operational demands of secure distribution can all shape continuity and stakeholder confidence. Organizations that respond with resilience-minded sourcing, manufacturing excellence, and compliance-first distribution practices will be better positioned to maintain dependable access.
Ultimately, leaders who align product strategy to real-world clinical workflows, tailor execution across channels and regions, and invest in governance capabilities can compete effectively while supporting responsible patient care. The most durable advantage will come from integrating clinical credibility, operational reliability, and policy readiness into a single, coherent strategy.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Tapentadol Tablets Market
Companies Mentioned
The key companies profiled in this Tapentadol Tablets market report include:- Alkem Laboratories Limited
- Amneal Pharmaceuticals, LLC
- Apotex Inc.
- Aristo Pharmaceuticals Pvt. Ltd.
- Aurobindo Pharma Limited
- Centurion Laboratories Private Limited
- Dr. Reddy's Laboratories Ltd.
- Eris Lifesciences Limited
- Grünenthal GmbH
- Hetero Drugs Limited
- Intas Pharmaceuticals Ltd.
- Ipca Laboratories Ltd.
- Janssen Pharmaceuticals, Inc.
- Klokter Life Sciences Pvt. Ltd.
- Lupin Limited
- Macleods Pharmaceuticals Ltd.
- Marksans Pharma Limited
- Medley Pharmaceuticals Limited
- Orient Pharma Limited
- Sandoz International GmbH
- Sun Pharmaceutical Industries Limited
- Synokem Pharmaceuticals Ltd.
- Teva Pharmaceutical Industries Ltd.
- Torrent Pharmaceuticals Ltd.
- Zydus Lifesciences Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
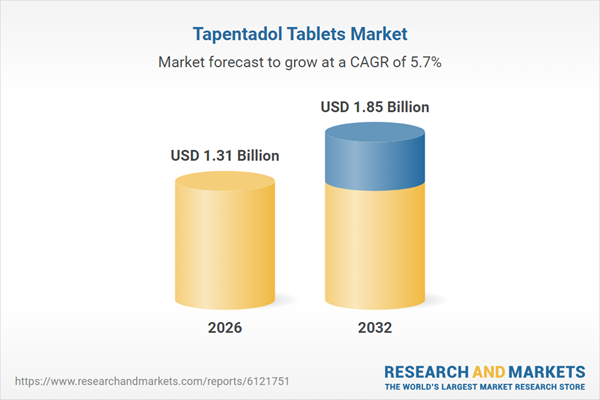
| Estimated Market Value ( USD | $ 1.31 Billion |
| Forecasted Market Value ( USD | $ 1.85 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |