Speak directly to the analyst to clarify any post sales queries you may have.
Embedding centers are becoming strategic workflow platforms as histology labs demand higher consistency, traceability, and ergonomic productivity
Paraffin embedding centers sit at the heart of histopathology and tissue-based research, translating fragile processed specimens into consistent, sectionable blocks that underpin diagnostic confidence and downstream molecular workflows. As laboratories face higher caseloads, tighter turnaround expectations, and intensifying scrutiny around traceability, the embedding station has evolved from a basic hot-and-cold workbench into an integrated productivity platform. Modern systems are expected to stabilize paraffin temperature precisely, support ergonomic workflow, reduce contamination risks, and integrate with labeling and tracking practices that protect specimen identity from grossing through microtomy.In parallel, the market conversation has shifted from simple “equipment replacement” to operational resilience. Labs are balancing standardization across multi-site networks with localized constraints such as bench space, power stability, staff availability, and maintenance support. This has increased interest in modular configurations that scale with demand, alongside a renewed appreciation for reliability and ease of service. As a result, the paraffin embedding center has become a strategic capital decision that influences quality management, labor efficiency, and the ability to implement consistent best practices.
Additionally, clinical and research workflows are converging in meaningful ways. The same labs that embed routine biopsies may also handle specialized immunohistochemistry workflows, small animal tissues, or biobank samples requiring rigorous documentation. This convergence raises expectations for embedding equipment to accommodate diverse cassette formats, variable tissue sizes, and controlled handling of paraffin and consumables. Consequently, the category is being redefined around controlled workflow, safety, and digital alignment rather than heating capacity alone.
Automation, digital traceability, sustainability demands, and procurement consolidation are redefining what laboratories expect from embedding centers
The competitive and operational landscape for paraffin embedding centers is being reshaped by a set of interlocking shifts that extend beyond incremental feature upgrades. First, automation is moving closer to the embedding bench. While full automation is not always practical given tissue variability and the nuanced judgments required during orientation, laboratories increasingly expect assisted workflows such as programmable temperature profiles, guided lighting, rapid response heating, and consistent cold-plate performance that reduce rework. These enhancements are not merely convenience features; they address staffing constraints and variability in training levels by making outcomes less dependent on individual technique.Second, digital readiness has become a proxy for long-term fit. Laboratories are tightening chain-of-custody practices, and embedding is a critical checkpoint where cassettes, molds, and blocks can be misidentified if labeling is inconsistent. As a result, demand is rising for embedding stations that support barcode-friendly layouts, offer accessory integration for printers or scanners, and align with laboratory information system workflows. Even when the embedding center itself is not networked, its physical design increasingly reflects the reality of digital traceability requirements.
Third, sustainability and safety expectations are influencing purchasing criteria. Energy consumption, heat management, and safe handling of paraffin are being discussed more actively, especially in facilities targeting environmental certifications or reducing operational costs. In addition, labs are paying closer attention to fume management, surface cleanability, and material durability to reduce contamination risk and improve cleaning turnaround. This is complemented by a broader shift toward preventive maintenance and serviceability, where buyers prioritize robust parts availability, intuitive diagnostics, and minimal downtime.
Finally, the procurement environment is changing. Consolidation among healthcare providers and the growth of large reference laboratories amplify the value of standardization across sites, negotiated service contracts, and predictable consumable usage. At the same time, smaller labs and specialty clinics often need flexible, space-efficient solutions that can be installed with minimal infrastructure changes. Taken together, these shifts are creating a market where vendors must compete on workflow outcomes, service performance, and integration readiness rather than on hardware specifications alone.
United States tariff changes in 2025 are altering sourcing strategies, lead times, and service continuity expectations across embedding center procurement
The introduction and expansion of United States tariff measures in 2025 has created tangible ripple effects across procurement planning for paraffin embedding centers, particularly where components, subassemblies, or finished units cross borders multiple times before final delivery. Because embedding stations blend heating elements, refrigeration or thermoelectric cooling modules, electronics, and precision-machined surfaces, tariff exposure can appear in several tiers of the bill of materials. This complexity has encouraged suppliers to re-examine sourcing footprints, renegotiate supplier terms, and evaluate partial localization strategies-changes that can influence lead times and configuration availability for end users.From the buyer’s perspective, the most immediate impact has been heightened variability in quoted pricing and delivery commitments. Many labs now face shorter quotation validity windows, more explicit freight and duties language, and a stronger push toward standardized configurations that are easier to manufacture and ship. In response, procurement teams are increasingly coordinating earlier with finance and compliance stakeholders to secure approvals, especially when a lab’s funding cycle or grant milestones are sensitive to delivery timing.
Operationally, tariffs have reinforced the value of service continuity and parts availability. If replacement boards, heating cartridges, or cold-plate components are subject to increased landed cost or import delays, downtime risk becomes more material. This has pushed some laboratories to emphasize domestic service coverage, local parts stocking, and clearer service-level agreements as part of the purchasing decision. Vendors, in turn, are differentiating through regional depots, interchangeable modules, and maintenance kits designed to reduce dependence on cross-border emergency shipments.
Over time, tariff-driven uncertainty is also shaping product strategy. Some manufacturers are evaluating design-for-substitution approaches, enabling alternative suppliers for key electronic components without compromising validation requirements. Others are exploring assembly closer to demand centers to reduce exposure and improve responsiveness. For the broader market, these changes elevate the importance of transparent total-cost evaluation and risk-adjusted procurement planning, where purchasing decisions consider not only upfront equipment pricing but also long-term operational resilience under shifting trade conditions.
Segmentation highlights show embedding-center demand is shaped by architecture, end-use workflow intensity, service models, and throughput complexity
Segmentation patterns in the paraffin embedding center category reveal that purchasing decisions are increasingly driven by how the equipment fits specific workflows rather than by broad lab-type labels. When viewed through the lens of product architecture, standalone embedding stations continue to appeal to laboratories that need simplicity, predictable operation, and easier validation pathways. However, integrated systems that combine heated work surfaces, dispensing control, and cold-plate coordination are gaining preference in environments where throughput and repeatability matter, because they reduce handoffs and make embedding steps more uniform across shifts.Looking at end-use settings, hospital pathology departments tend to prioritize reliability, safety features, and compatibility with standardized consumables because embedding is a daily, high-stakes activity tied to diagnostic turnaround. Reference laboratories and multi-site networks often emphasize standardization, service agreements, and ease of training, since embedding outcomes must be consistent across teams and locations. Academic and research institutions typically require flexibility to accommodate diverse specimen types, experimental protocols, and intermittent high-volume periods, which increases interest in modular accessories and adjustable temperature control.
Workflow-driven differentiation also emerges when considering throughput needs. Lower-volume labs often choose compact, cost-conscious configurations that still deliver stable temperature performance and easy cleaning, because staff may multitask across histology roles. Higher-volume operations lean toward larger work areas, faster paraffin recovery, and cold-plate capacity to handle batch embedding without bottlenecks. In these settings, small ergonomic gains-like improved lighting, intuitive control placement, and reduced reach distances-translate into meaningful productivity and reduced error risk.
Finally, purchasing behavior differs based on acquisition and service preferences. Some laboratories favor bundled procurement models where the embedding center is purchased alongside microtomes, tissue processors, and service coverage to streamline vendor management. Others prefer best-of-breed sourcing and negotiate service separately to optimize performance and cost across the workflow. Across these segmentation views, a clear throughline is emerging: laboratories want embedding centers that reduce variability, support traceability practices, and remain maintainable under staffing and supply-chain constraints.
Regional buying patterns diverge by infrastructure maturity, consolidation, and service readiness across the Americas, EMEA, and Asia-Pacific ecosystems
Regional dynamics in the paraffin embedding center landscape reflect differences in healthcare infrastructure, lab consolidation, regulatory emphasis, and procurement models. In the Americas, replacement cycles are often influenced by consolidation among provider networks and the rise of centralized reference testing, which increases demand for standardized equipment fleets and reliable service coverage. Buyers frequently weigh service responsiveness and parts availability heavily, particularly when embedding is a critical step supporting rapid diagnostic turnaround.Across Europe, the Middle East, and Africa, purchasing criteria vary widely between mature Western European lab networks and emerging capacity builds in parts of the Middle East and Africa. In mature systems, there is strong attention to safety, cleanability, and quality management alignment, including documentation practices that reduce identification risk. Meanwhile, facilities investing in new or expanded histology capability often seek robust, easy-to-train equipment that can operate reliably under variable staffing conditions and that can be supported through dependable distributor and service networks.
In Asia-Pacific, growth in diagnostic testing capacity, expansion of private laboratory chains, and increased investment in hospital infrastructure are shaping demand for both value-oriented and advanced configurations. Large urban centers may adopt higher-capacity embedding setups to support rising caseloads and specialization, while smaller cities and regional facilities often prioritize compact footprints and cost-effective performance. The region also exhibits strong sensitivity to delivery timelines and service coverage, as multi-country supply chains and diverse regulatory environments can complicate procurement.
Across all regions, one unifying trend is the stronger role of total lifecycle considerations. Laboratories increasingly compare not just hardware capabilities but also the availability of training, the clarity of maintenance procedures, and the resilience of supply for consumables and spare parts. As a result, regional go-to-market success depends on aligning product configurations with local workflow realities while ensuring service infrastructure can sustain consistent performance after installation.
Company differentiation is shifting toward service reliability, ergonomic workflow design, configurable platforms, and supply-chain resilience under scrutiny
Company strategies in the paraffin embedding center space are converging around measurable workflow outcomes, yet differentiation remains clear in how suppliers prioritize usability, durability, and serviceability. Leading manufacturers tend to emphasize stable thermal control and robust cold-plate performance as foundational requirements, then layer on ergonomic enhancements such as improved illumination, intuitive user interfaces, and layouts that reduce repetitive motion. These design priorities reflect a broader understanding that embedding is both technical and human-centered, where fatigue and bench organization can directly affect quality.Another axis of differentiation is how companies support operational continuity. Suppliers with strong service networks invest in regional parts depots, technician training, and preventive maintenance programs that reduce downtime. In procurement discussions, buyers increasingly reward vendors that can articulate clear maintenance schedules, provide rapid replacement pathways for critical modules, and offer documentation that supports quality audits. This is particularly important for laboratory networks seeking consistent performance across multiple sites.
Companies are also refining portfolio strategy by offering configurable platforms rather than one-size-fits-all stations. Optional accessories, interchangeable cold plates, different paraffin dispensing approaches, and add-on safety features allow vendors to serve both high-throughput histology centers and smaller specialty labs without forcing unnecessary complexity. This configurability supports broader channel strategies as well, enabling distributors and direct sales teams to tailor solutions to local facility constraints.
Finally, competitive positioning is being influenced by supply-chain resilience and compliance readiness. Vendors that can demonstrate stable component sourcing, consistent product availability, and transparent change-control practices have an advantage in environments where procurement committees scrutinize risk. As trade policy uncertainty persists and labs demand predictable uptime, company credibility is increasingly built on execution-delivering consistent performance, dependable support, and validated continuity-not solely on feature innovation.
Leaders can raise embedding quality and throughput by focusing on workflow mapping, traceability safeguards, service resilience, and workforce-ready design
Industry leaders can strengthen outcomes in paraffin embedding by treating the embedding center as part of an end-to-end system rather than an isolated bench purchase. Start by mapping the current-state workflow from tissue processing to microtomy and identifying where embedding creates delays, rework, or identification risk. This process often reveals that the highest return comes from small operational improvements-such as bench layout standardization, temperature setpoint governance, and consistent mold and cassette handling-supported by equipment that makes the preferred process easy to follow.Next, prioritize traceability and error prevention in procurement criteria. Embedding is a common point where cassettes, molds, and blocks converge, so leaders should require physical layouts and accessory compatibility that support barcode-forward workflows, clear labeling visibility, and clean handoffs. Where appropriate, add procedural controls such as standardized block face orientation conventions and second-check steps for high-risk specimens, ensuring the equipment setup enables these practices without slowing throughput.
Leaders should also adopt a resilience-first approach to service and parts planning. Evaluate vendors not only on warranty language but on practical service coverage, typical response times, and the availability of critical spare parts. Consider negotiating preventive maintenance schedules aligned to workload intensity and building an internal readiness plan that includes staff training refreshers, cleaning protocols, and documented troubleshooting steps to reduce avoidable downtime.
Finally, align embedding investments with workforce realities. With staffing shortages and variable experience levels in many labs, training simplicity and ergonomic design become strategic. Select systems with intuitive controls, stable performance, and easy cleaning, then formalize competency-based training supported by clear visual work instructions. Over time, this combination of well-chosen equipment and disciplined process management can reduce variability, improve turnaround consistency, and protect quality under changing demand.
A triangulated methodology blends stakeholder interviews, technical documentation review, and segmentation validation to mirror real lab purchasing behavior
The research methodology for assessing the paraffin embedding center landscape combines structured primary inquiry with rigorous secondary analysis to ensure insights reflect real purchasing behavior and operational constraints. Primary research typically includes interviews with laboratory managers, histotechnologists, procurement stakeholders, and service professionals to understand workflow pain points, decision criteria, and post-installation performance expectations. These conversations are designed to capture the practical realities of embedding-from daily cleaning burdens to the impact of downtime on turnaround targets.Secondary research consolidates publicly available information such as regulatory frameworks, trade and customs developments, product documentation, corporate filings, patent activity, and tender patterns where accessible. This step helps contextualize how suppliers position their systems, how compliance requirements influence equipment design, and how supply-chain changes can affect availability and service support.
To maintain consistency, findings are triangulated across stakeholder perspectives and validated against observable market signals such as product refresh cycles, distribution expansions, and service infrastructure investments. The methodology also applies a structured segmentation framework to compare needs across different lab environments and throughput profiles, ensuring that insights translate into actionable procurement and strategy guidance.
Quality control is supported through iterative review, where contradictions are reconciled by follow-up inquiry and careful source evaluation. This approach prioritizes factual accuracy, avoids overreliance on any single viewpoint, and produces a balanced assessment of technology direction, buyer priorities, and operational risk factors shaping the paraffin embedding center category.
Embedding-center selection is evolving into a resilience-focused workflow investment that protects quality, throughput, and traceability under change
Paraffin embedding centers are increasingly recognized as high-leverage infrastructure in histology, influencing not only block quality but also turnaround consistency, staff efficiency, and traceability integrity. The market is moving toward systems that reduce variability through stable thermal performance, ergonomic workflow support, and compatibility with modern identification practices. At the same time, laboratories are raising expectations for cleanability, safety, and lifecycle service readiness.As procurement becomes more risk-aware-shaped by staffing constraints, supply-chain volatility, and evolving trade policies-buyers are shifting from feature-first comparisons to resilience-first evaluation. This favors suppliers that can demonstrate dependable support, transparent maintenance pathways, and configurable platforms that adapt to diverse lab settings.
Ultimately, the embedding center decision is best approached as a strategic workflow investment. Organizations that align equipment selection with standardized process design, training discipline, and service continuity planning will be better positioned to sustain quality and throughput, even as clinical demand and operational complexity continue to rise.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Paraffin Embedding Center Market
Companies Mentioned
The key companies profiled in this Paraffin Embedding Center market report include:- Amos Scientific
- Bio-Optica Milano
- Celnovte Biotechnology Co., Ltd.
- Diapath S.p.A.
- Histo-Line S.r.l.
- Jinhua Kaindi Medical Devices Co., Ltd.
- Leica Biosystems Nussloch GmbH
- LUPETEC
- Medite GmbH
- Micros Produktions-u.HandelsgmbH.
- Microteknik
- Milestone S.r.l.
- Neolab Migge GmbH
- Sakura Finetek U.S.A., Inc.
- Sipcon Technologies Private Limited
- SLEE Medical GmbH
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
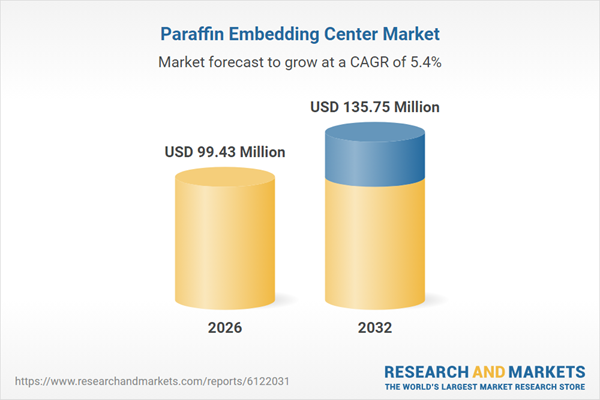
| Estimated Market Value ( USD | $ 99.43 Million |
| Forecasted Market Value ( USD | $ 135.75 Million |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |