Speak directly to the analyst to clarify any post sales queries you may have.
Why the humble 3-way stopcock is becoming a strategic linchpin for safer fluid management, procedural efficiency, and supply assurance in care delivery
Medical 3-way stopcocks are deceptively simple components that sit at the center of modern fluid management, enabling controlled infusion, aspiration, pressure monitoring, and medication delivery through a compact valve interface. Their clinical role spans anesthesia, critical care, cardiology, radiology, oncology, dialysis, and general inpatient therapy, where reliable flow direction and secure connections reduce workflow friction and help clinicians maintain sterility and dosing accuracy. As hospitals and ambulatory centers standardize sets and protocols, the stopcock increasingly functions as an interoperability node within broader IV administration and monitoring ecosystems.While the core mechanism has long been established, expectations around safety, usability, and traceability continue to rise. Infection prevention programs increasingly scrutinize every touchpoint in the fluid path, and device committees demand clearer evidence of compatibility with connectors, catheters, and extension lines. At the same time, procurement teams look for resilience against supply disruption and volatility in polymers, resins, and specialized elastomers.
Against this backdrop, competitive advantage depends less on the basic valve concept and more on execution: material science that resists cracking and chemical stress, designs that support one-handed operation and clear flow visualization, manufacturing consistency that minimizes torque variability, and packaging configurations that fit standardized procedure kits. Therefore, an executive view of this market must connect clinical drivers, regulatory expectations, and supply-chain realities into a coherent picture that informs portfolio, sourcing, and commercialization choices.
How infection control priorities, systemwide standardization, human-factors design, and resilience-first sourcing are reshaping competition for stopcocks
The landscape is shifting from commodity purchasing toward clinically anchored standardization, driven by hospital system consolidation and a stronger emphasis on protocol-driven care. As integrated delivery networks harmonize IV policies across sites, they increasingly specify connector types, preferred set configurations, and performance expectations that suppliers must meet consistently. This favors manufacturers that can offer stable quality, broad compatibility, and documentation that simplifies value analysis reviews.In parallel, infection prevention has moved from an episodic compliance concern to a continuous operational mandate. Scrutiny of open ports, backflow potential, and touch contamination risk is influencing preference for designs that pair effectively with closed systems, needle-free connectors, and disinfection caps. Although stopcocks are not always the primary focus of infection initiatives, they are being evaluated more rigorously as part of the overall fluid pathway where a single weak link can undermine best practices.
Another shift is the elevation of human factors and visualization. Facilities seek clearer markings, smoother actuation, and tactile feedback that supports rapid decision-making under stress, especially in critical care and emergency contexts. Manufacturers are responding with improved handle geometry, higher-contrast indicators, and materials that maintain clarity under common disinfectants and lipids.
Finally, the industry is absorbing lessons from recent supply disruptions. Dual sourcing, regional manufacturing footprints, and greater transparency on origin and materials are increasingly valued in tenders. As a result, competitive differentiation is expanding beyond product features into logistics reliability, regulatory readiness across jurisdictions, and the ability to support customized kitting and labeling requirements without compromising lead times.
What United States tariff pressures in 2025 mean for stopcock sourcing, upstream materials, and contract structures in cost-sensitive disposable supply chains
United States tariff dynamics in 2025 are exerting pressure on procurement and pricing strategies for components and finished disposable devices that rely on globally traded polymers, precision molds, and subassemblies. Even when the stopcock itself is assembled domestically, upstream exposure can persist through resin inputs, tooling, packaging materials, and connector components that cross borders multiple times before final conversion. This layered supply chain structure makes tariff impacts uneven and sometimes difficult to isolate in line-item negotiations.In response, many suppliers are strengthening country-of-origin documentation and revisiting bill-of-materials architectures to reduce the tariffable content of finished goods. Some are exploring alternate resin grades or qualifying secondary sources for critical parts, although material substitutions must be balanced against requirements for biocompatibility, transparency, and resistance to stress cracking from alcohol-based disinfectants and lipid-containing infusions. For regulated medical devices, the qualification burden can be substantial, so the tariff response tends to favor redesign-for-manufacture, supplier diversification, and process localization rather than rapid material switching.
Buyers are also adapting. Hospital contracting teams increasingly request clearer price adjustment mechanisms and continuity plans, while distributors and kit assemblers seek predictable input costs to maintain stable procedure-pack pricing. This environment rewards suppliers that can offer contractual clarity, contingency inventory strategies, and evidence of validated alternates.
Over time, these tariff pressures may accelerate a broader rebalancing toward regionalized manufacturing and shorter supply lines for high-volume disposables. However, because stopcocks are cost-sensitive, the transition is likely to be incremental and closely tied to automation investments, scale efficiencies, and the ability to maintain consistent valve torque and leak performance during process transfers.
Segmentation patterns that reveal how pressure rating, configuration, materials, sterilization choices, end users, and channels shape real-world adoption and risk
Across product type, 3-way stopcocks that are high-pressure rated tend to align more closely with imaging and interventional workflows where injector compatibility and burst resistance are non-negotiable. In contrast, standard-pressure variants remain prevalent in general infusion and routine monitoring, where ease of use and cost discipline dominate decisions. This split reinforces the need for portfolio clarity, because performance claims and validation expectations differ meaningfully between use contexts.When viewed through configuration, single stopcocks often serve as flexible, on-demand add-ons in bedside care, while extension-set-integrated offerings support streamlined setups and reduce connection steps in time-sensitive environments. Manifold formats, in turn, map to complex multi-line therapies and ICU workflows where centralized control reduces line clutter. Each configuration influences not only clinical convenience but also the number of connection points, which is increasingly considered through an infection prevention lens.
Material segmentation highlights trade-offs that matter operationally. Polycarbonate continues to be valued for clarity and strength but can face scrutiny around chemical compatibility and stress cracking, especially under aggressive disinfection routines. Polypropylene is often positioned for chemical resistance and cost effectiveness, though it may present different tactile and transparency characteristics. Acrylic and other engineered materials appear in niche designs where specific optical or mechanical properties are prioritized. These material choices intersect with sterilization method and shelf-life expectations, shaping how suppliers justify selection and how buyers evaluate total risk.
Sterilization method further differentiates offerings because ethylene oxide sterilization supports many polymer assemblies but is increasingly scrutinized for emissions management and compliance rigor. Gamma and e-beam approaches can streamline certain validation paths yet may affect polymer properties and long-term clarity depending on formulation. Consequently, sterilization is no longer a back-end detail; it is a strategic attribute that influences procurement confidence and regulatory readiness.
End-user segmentation underscores distinct buying behaviors. Hospitals and clinics often purchase through tenders and value analysis committees that prioritize standardization, while ambulatory surgical centers emphasize setup speed and predictable kit contents. Diagnostic centers and imaging suites focus on pressure performance and compatibility with injectors and contrast media protocols. Home care and long-term care settings, where applicable, prioritize simplicity and caregiver usability. Meanwhile, distribution and kit assemblers require consistency, packaging flexibility, and dependable fill rates to support downstream service levels.
Channel dynamics also matter. Direct sales models can support customization and clinical education, whereas distributors provide reach and contract coverage. E-commerce and digital procurement are emerging as supplemental channels, particularly for smaller facilities and urgent replenishment, though regulatory documentation and lot traceability expectations remain central. Altogether, segmentation reveals a market where operational fit, validation strength, and supply assurance determine success as much as the valve itself.
Regional realities that determine purchasing behavior and adoption, from protocol-driven systems in the Americas to compliance nuance in Europe and growth markets beyond
In the Americas, purchasing is strongly shaped by integrated delivery networks, group purchasing organizations, and a high level of protocol standardization that elevates the importance of documentation, consistency, and contingency supply. Clinical emphasis on infection prevention and needle-free compatibility reinforces demand for designs that integrate cleanly into closed-system approaches, while tariff and logistics considerations intensify attention on origin transparency and multi-site availability.Across Europe, regulatory alignment and sustainability-driven procurement are increasingly influential. Buyers often expect robust technical files, clear labeling, and evidence of conformity with evolving requirements, alongside growing interest in packaging reduction and responsible sterilization practices. The region’s diverse tender structures also reward suppliers that can navigate country-level nuances and provide stable supply without fragmenting product platforms.
In the Middle East & Africa, investment in hospital infrastructure and expanding access to advanced care are creating opportunities for reliable disposables that support ICU expansion, surgical volumes, and dialysis capacity. At the same time, supply continuity and distributor capability are frequently decisive, with purchasers valuing partners who can ensure training, documentation, and dependable replenishment across varying import and regulatory pathways.
Asia-Pacific reflects a mix of high-growth healthcare demand and rapidly modernizing procurement. Large markets are increasing expectations for quality systems, traceability, and local responsiveness, while competitive pricing remains important. Regional manufacturing scale, strong distributor networks, and the ability to tailor packaging and labeling for different jurisdictions help suppliers win share. Moreover, rising procedural volumes in interventional radiology and cardiology elevate the relevance of high-pressure rated stopcocks and compatible accessories.
Taken together, regional insights point to a common theme: success depends on pairing globally consistent quality and validation with locally relevant contracting, logistics, and compliance support. Suppliers that treat regions as operational ecosystems rather than just sales territories are better positioned to meet both clinical and commercial expectations.
What separates leading stopcock suppliers today: repeatable performance, integrated portfolios, manufacturing discipline, and credibility as a risk-management partner
Competition among key companies is increasingly defined by the ability to deliver consistent valve performance at scale while meeting rising expectations for compatibility, traceability, and documentation. Leading suppliers differentiate through tight process control that minimizes variability in torque, leakage, and dead space, because these attributes directly affect clinician confidence and downstream outcomes in high-acuity environments.Another area of separation is portfolio breadth and integration. Companies that can supply complementary components such as extension lines, needle-free connectors, manifolds, and procedure kits reduce the burden on procurement teams and simplify standardization. This integrated approach is especially attractive to health systems looking to reduce SKU proliferation while maintaining flexibility across departments.
Manufacturing maturity is also a clear divider. Firms with advanced molding capabilities, in-line inspection, and validated assembly processes can sustain higher throughput without compromising dimensional tolerances that drive reliable luer engagement. In addition, companies that have invested in packaging engineering and labeling automation can support private labeling, country-specific compliance markings, and kit-friendly configurations while maintaining lot-level traceability.
Finally, commercial credibility increasingly depends on supply resilience and quality responsiveness. Buyers pay close attention to how quickly a supplier investigates complaints, implements corrective actions, and communicates changes in materials or processes. In a market where the product is small but mission-critical, the strongest companies behave like risk-management partners rather than commodity vendors, supporting both clinical education and procurement assurance.
Practical moves leaders can take now to win tenders and trust: platform validation, resilient sourcing, standardization support, and credible sustainability execution
Industry leaders should prioritize design and validation choices that align with the strictest real-world use cases, then scale downward without fragmenting the platform. For many portfolios, this means ensuring robust performance under pressure, chemical exposure, and repeated manipulation, while maintaining clear flow indication and predictable actuation. Investing in human factors validation can reduce downstream adoption friction, particularly in environments where speed and clarity are essential.Supply-chain strategy should be treated as a product feature. Qualifying dual sources for critical inputs, improving transparency on origin, and building pragmatic safety stock policies can stabilize service levels in the face of tariff shifts and logistics volatility. Where feasible, leaders can evaluate regional manufacturing or final assembly options to reduce upstream exposure, but they should pair these moves with rigorous process transfer controls to avoid performance drift.
Commercially, aligning with customer standardization goals is a practical growth lever. Leaders can offer rationalized SKU sets, cross-department compatibility guides, and kit-ready packaging options that support anesthesia, ICU, and interventional workflows without forcing facilities into unnecessary variation. Additionally, clear change-notification practices and readily accessible technical documentation can accelerate value analysis approvals and strengthen long-term contracts.
Finally, sustainability and compliance should be advanced through measurable, operations-backed initiatives. Packaging optimization, responsible sterilization stewardship, and transparent materials disclosure can support procurement requirements without overpromising. The most effective approach ties environmental improvements to supply reliability and quality outcomes, creating a narrative that resonates with both clinicians and executives.
A transparent research approach that blends stakeholder interviews, regulatory and technical review, and triangulation to produce decision-grade market understanding
The research methodology combines structured primary engagement with rigorous secondary review to ensure insights reflect both clinical realities and operational purchasing constraints. Primary inputs are gathered through interviews and discussions with stakeholders across the value chain, including clinicians, infection prevention personnel, supply-chain and procurement leaders, distributors, and manufacturing and quality professionals. These conversations focus on decision criteria, workflow pain points, validation expectations, and evolving preferences around connectors, sterilization, and packaging.Secondary research synthesizes information from regulatory publications, standards organizations, tender and procurement frameworks, company disclosures, clinical practice guidance, and technical literature related to polymer performance, sterilization effects, and connector compatibility. This step is used to contextualize primary insights within the broader compliance environment and to track how policy changes and enforcement trends influence purchasing behavior.
Data triangulation is applied to reconcile differences between stakeholder perspectives, cross-check product claims against documented requirements, and identify themes that persist across regions and care settings. The approach emphasizes traceability of assumptions, ensuring that conclusions about competitive differentiation, adoption drivers, and procurement priorities are grounded in repeatable evidence.
Finally, findings are curated into an executive narrative that connects segmentation, regional dynamics, and risk factors into actionable implications. The goal is to provide decision-makers with a clear line of sight from clinical needs to operational execution, supporting portfolio planning, supplier strategy, and go-to-market alignment.
Bringing the story together: stopcocks are small components with outsized clinical and operational consequences in an era of scrutiny and disruption
Medical 3-way stopcocks remain foundational to safe and efficient fluid management, yet the factors that determine purchasing and preference are becoming more complex. Clinical priorities around infection prevention, compatibility, and human factors are rising in prominence, and they increasingly intersect with operational concerns such as documentation burden, standardization, and supply continuity.At the same time, tariff-related cost pressure and broader supply-chain volatility are pushing both buyers and suppliers toward greater transparency and resilience. This is accelerating attention to upstream materials, country-of-origin clarity, and validated alternates, all while maintaining strict performance expectations in high-pressure and high-acuity use cases.
Ultimately, the market rewards companies that treat stopcocks as mission-critical components within larger therapy and monitoring systems. Those that deliver consistent performance, simplify customer standardization, and operate with disciplined quality and responsive service will be best positioned to earn long-term trust and sustainable adoption across regions and care settings.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Medical 3-Way Stopcock Market
Companies Mentioned
The key companies profiled in this Medical 3-Way Stopcock market report include:- Argon Medical Devices, Inc.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton Dickinson and Company
- Bicakcilar
- Cardinal Health, Inc.
- CV Medica
- Demax
- Demax Medical
- Elcam Medical
- Fresenius Kabi AG
- ICU Medical, Inc.
- JCM MED
- KYOLING
- Medline Industries, Inc.
- Merit Medical Systems, Inc.
- Nipro Corporation
- Pfizer Inc.
- Poly Medicure Ltd.
- Shunmei Medical
- Smiths Group plc
- Teleflex Incorporated
- Terumo Corporation
- Vygon SAS
- World Precision Instruments
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
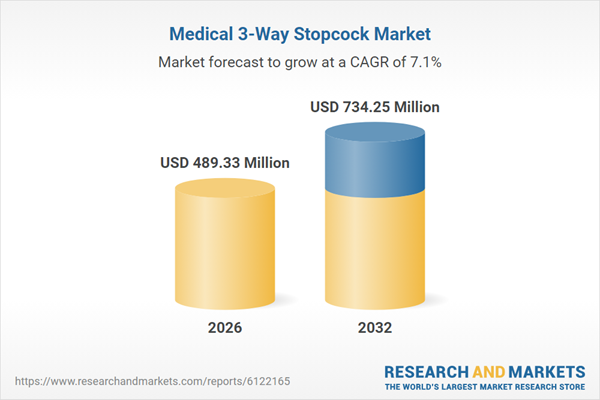
| Estimated Market Value ( USD | $ 489.33 Million |
| Forecasted Market Value ( USD | $ 734.25 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |