Speak directly to the analyst to clarify any post sales queries you may have.
A clear, decision-ready introduction to the α-Ketoacid API landscape where regulated quality, metabolic science, and supply resilience converge
α-Ketoacid active pharmaceutical ingredients (APIs) sit at the intersection of chemistry, clinical nutrition, and regulated manufacturing, serving applications where metabolic pathways and nitrogen balance matter as much as purity and reproducibility. In practice, these APIs are often associated with therapeutic nutrition strategies, including formulations used in chronic kidney disease management, as well as with adjacent pharmaceutical and specialty chemical use cases that depend on consistent stereochemistry and tight impurity controls.What makes the α-ketoacid API landscape distinctive is the combination of stringent quality expectations and complex supply-chain realities. Starting materials can be sensitive to commodity pricing and availability, synthesis routes can require careful control of intermediates, and finished APIs must meet demanding specifications that influence downstream formulation performance. Consequently, buyers increasingly evaluate suppliers not only on certificate-of-analysis results, but also on process robustness, deviation management, audit transparency, and the ability to maintain continuity during logistics disruption.
As stakeholders plan for the next phase of growth and compliance, it becomes essential to understand how innovation in manufacturing, shifts in global sourcing, and evolving regulatory and trade dynamics are reshaping competitive advantage. This executive summary frames those forces, highlighting where strategic choices in segmentation, region, and company capabilities can most materially improve outcomes.
Transformative shifts redefining α-Ketoacid APIs: capability-based sourcing, greener process control, and supply-chain redesign for resilience
The α-ketoacid API market is experiencing a shift from price-first procurement to capability-led sourcing, driven by higher expectations for traceability, documentation completeness, and repeatable process performance. Buyers are increasingly aligning vendor selection with risk frameworks that assess not just batch compliance, but also the maturity of quality systems, the stability of critical raw material inputs, and the supplier’s readiness for regulatory inspections and customer audits.In parallel, manufacturing strategies are becoming more differentiated. Process intensification, improved chiral control, and solvent management are being used to enhance yield and reduce impurity formation, while greener chemistry principles are shaping how companies evaluate route selection and waste profiles. This matters because α-ketoacid APIs can face tight acceptance criteria for residual solvents and related substances, and small improvements in process control can translate into meaningful advantages in consistency and cost-to-serve.
Another transformative shift is the rebalancing of global supply chains. Customers are increasingly seeking dual sourcing, regional redundancy, and clearer provenance of key inputs, especially where single-country dependency has previously dominated. As a result, firms with flexible manufacturing networks, validated alternate routes, and strong supplier qualification programs are better positioned to respond when shipping lanes tighten, energy prices fluctuate, or local regulatory expectations change.
Finally, collaboration models are evolving. Instead of transactional purchasing, many buyers now prefer structured technical partnerships that include joint change-control planning, stability data sharing, and proactive communication on deviations. This shift rewards API manufacturers that can operate with a “customer quality” mindset, anticipating questions from formulators and regulators and providing rapid, well-documented responses that keep downstream timelines intact.
Cumulative impact of United States tariffs in 2025 on α-Ketoacid APIs: landed-cost volatility, sourcing redesign, and compliance-driven delays
United States tariff actions anticipated in 2025 have the potential to reshape landed-cost economics for selected chemical and pharmaceutical inputs, with implications that extend beyond straightforward price increases. For α-ketoacid APIs and their precursors, tariffs can influence supplier selection, contracting structures, and even technical decisions about synthesis routes when certain intermediates become less economical to import.One of the most immediate impacts is procurement behavior that shifts from spot buying to longer-term contracting with contingency clauses. Importers tend to seek pricing stability, clearer rules for tariff pass-through, and alternative Incoterms arrangements to manage volatility. At the same time, compliance teams often intensify documentation requirements to confirm accurate classification and country-of-origin determinations, because errors can trigger delays, retroactive duties, and reputational risk during audits.
Tariffs can also catalyze changes in supply-chain architecture. Some organizations respond by qualifying secondary suppliers in tariff-neutral regions, while others pursue partial localization, such as domestic finishing steps, reprocessing, or packaging that may simplify logistics and reduce exposure. However, these approaches are constrained by regulatory expectations: any meaningful process change may require validation, robust comparability assessments, and alignment with customer change-control timelines. As a result, companies that already maintain validated alternates and well-documented process descriptions can adjust faster than those starting from a single-source baseline.
Downstream customers may see impacts in lead times as well as costs. When tariffs alter import flows, capacity can tighten in non-tariff regions as demand concentrates, increasing the importance of demand planning, safety-stock strategies, and transparent allocation policies. In response, leading suppliers are expected to differentiate through predictable fulfillment, clear communication, and disciplined batch release timelines-capabilities that help customers maintain continuity even when trade policy introduces friction.
Over time, tariff pressure may accelerate investment in regional manufacturing and more diverse sourcing of key reagents. Yet the benefits will accrue unevenly: organizations with strong regulatory readiness, scalable quality systems, and proven technical transfer capabilities can convert policy disruption into a competitive advantage, while those with limited documentation depth may struggle to qualify alternates quickly enough to protect customer supply.
Key segmentation insights for α-Ketoacid APIs across product types, applications, grades, end users, and channels shaping qualification and value
Segmentation dynamics in α-ketoacid APIs are best understood by examining how product specificity, performance expectations, and buying criteria differ across use cases and procurement pathways. When considering the market through the lens of product type, distinctions among keto analogs such as keto-leucine, keto-isoleucine, keto-valine, keto-phenylalanine, and keto-methionine drive different synthesis and control strategies, particularly around stereochemistry, impurity profiles, and stability behavior. These differences shape how manufacturers prioritize analytical method development and how buyers evaluate supplier competence.Equally important is segmentation by application. Demand patterns and qualification pathways diverge between pharmaceutical use, clinical nutrition and medical food use, and research or specialty chemical use. In clinical nutrition settings, the emphasis often falls on consistent performance in formulations, tight heavy metal limits, and reliable supply for long treatment regimens, while pharmaceutical pathways may require deeper documentation, formal change control, and broader stability packages. Research and specialty applications can be more flexible in volumes yet demanding in customization, encouraging suppliers to offer smaller batch sizes and faster technical support.
Segmentation by grade further influences supplier selection and pricing power. Pharmaceutical-grade requirements tend to elevate expectations for GMP maturity, audit responsiveness, and validated analytical procedures. Food or nutraceutical-grade requirements can remain stringent, but the buyer’s evaluation often weighs different risk considerations, including allergen statements, microbial limits, and suitability for specific formulation conditions. This separation by grade effectively creates distinct competitive arenas, where quality system depth and documentation readiness become differentiators beyond basic chemical synthesis capability.
The landscape also varies based on end-user segmentation, including hospitals and clinics, contract manufacturers, and branded product companies. Hospitals and clinics often prioritize continuity, standardized documentation, and consistent supply programs, while contract manufacturers may focus on supplier responsiveness, lead time reliability, and the ability to support rapid scaling. Branded product companies, in turn, frequently seek suppliers that can support marketing claims through robust traceability, quality narratives, and sustainability-aligned practices.
Finally, segmentation by distribution channel-direct sales versus distributors-affects how quickly technical information flows and how issues are resolved. Direct relationships can accelerate qualification and change-control alignment, whereas distributor-led models can broaden reach but may add layers that slow technical exchanges unless the supplier maintains strong documentation portals and clear escalation pathways. Across all segmentation dimensions, the most successful strategies align product and grade capabilities with the application’s regulatory burden and the end user’s tolerance for change, creating a coherent go-to-market approach rather than a one-size-fits-all offering.
Key regional insights for α-Ketoacid APIs across the Americas, EMEA, and Asia-Pacific as compliance, capacity, and logistics reshape demand
Regional dynamics for α-ketoacid APIs are shaped by the intersection of regulatory expectations, manufacturing ecosystems, and procurement risk preferences. In the Americas, buyer priorities often center on supply assurance, documentation completeness, and transparent quality systems that support audits and ongoing oversight. This creates opportunities for suppliers with strong customer-facing quality functions, responsive deviation management, and the ability to provide robust traceability packages that withstand scrutiny.Across Europe, the Middle East, and Africa, regulatory rigor and sustainability expectations can be particularly influential in supplier evaluation. Buyers may place heightened emphasis on consistent GMP performance, strong environmental controls, and well-supported change management. Additionally, a diverse set of healthcare and reimbursement environments can shape product adoption pathways, making it essential for suppliers to tailor documentation and technical support to different procurement frameworks and clinical practice norms.
In Asia-Pacific, the region’s manufacturing depth and supply-chain scale play a decisive role, with strong capabilities in chemical synthesis and intermediates production supporting broad availability. At the same time, customers increasingly differentiate among suppliers based on demonstrated quality maturity, inspection readiness, and the ability to meet international documentation standards. This has led to greater investment in analytical infrastructure, digital quality records, and upgraded facilities to support global customer requirements.
Taken together, regional insights point to a more interconnected market where cross-border qualification is common but not frictionless. Logistics constraints, evolving trade policies, and variable inspection expectations can amplify the value of regional redundancy and dual sourcing. Companies that can offer aligned specifications, consistent analytical methods, and harmonized documentation across multiple sites are better positioned to serve multinational customers seeking stability across regions without compromising compliance or performance.
Key company insights in α-Ketoacid APIs where quality systems, technical transfer strength, transparency, and sustainable operations define leadership
Competition in α-ketoacid APIs increasingly hinges on execution excellence across manufacturing consistency, analytical rigor, and customer responsiveness. Leading companies distinguish themselves by maintaining stable processes with well-controlled impurity profiles, investing in advanced analytical techniques to detect and trend low-level contaminants, and building disciplined quality systems that can support frequent customer audits.Another differentiator is technical transfer capability. Organizations with repeatable scale-up playbooks, validated alternate raw materials, and documented process knowledge can support customers through formulation changes, site shifts, and regulatory submissions with fewer delays. This is particularly important when trade policies, logistics disruptions, or capacity constraints force buyers to consider alternate sources or adjust their supply chains.
Commercial strength is also shaped by how companies manage relationships and transparency. Suppliers that proactively communicate on lead times, capacity planning, and potential constraints build trust that can translate into longer-term agreements. In addition, firms that provide comprehensive documentation-covering traceability, stability, impurity rationale, and change-control commitments-reduce friction in qualification and enable faster onboarding by regulated customers.
Finally, broader operational maturity matters as much as chemistry. Companies that integrate EHS discipline, solvent recovery, waste management, and energy efficiency into their operating models can better meet evolving customer expectations around sustainability and responsible manufacturing. As procurement teams increasingly evaluate total supplier risk, these capabilities help differentiate credible long-term partners from purely transactional vendors.
Actionable recommendations for α-Ketoacid API leaders to build tariff-ready supply chains, audit-proof quality, and segment-aligned growth strategies
Industry leaders can strengthen their position by treating supply resilience as a design principle rather than a contingency plan. That starts with qualifying alternate sources of critical raw materials, validating secondary manufacturing routes where feasible, and building a structured playbook for change control that anticipates customer documentation needs. By doing so, companies can respond faster to tariff shifts, shipping disruption, or sudden capacity constraints without triggering avoidable qualification delays.Next, leaders should deepen analytical and documentation capabilities in ways that directly reduce customer friction. Expanding impurity characterization, tightening method validation, and improving lot-to-lot trending can help prevent deviations and accelerate investigations when anomalies occur. In regulated contexts, speed and clarity of response matter; investing in documentation workflows, data integrity controls, and audit readiness can become a commercial advantage.
Commercial strategy should also align with segmentation realities. Suppliers can improve win rates by tailoring value propositions to application and grade requirements, offering service levels that reflect end-user risk tolerance, and clarifying the technical support model for direct and distributor channels. In many cases, disciplined portfolio focus-prioritizing products where the organization can reliably meet the highest expectations-outperforms overly broad catalogs that strain quality and operations.
Finally, leaders should prepare for trade and policy variability by adopting tariff-aware contracting and scenario planning. Structuring agreements with transparent cost drivers, building regional inventory strategies, and maintaining flexible logistics options can stabilize customer experience even when external conditions change. When combined with credible sustainability initiatives and responsible manufacturing practices, these steps help companies earn preferred-supplier status and secure longer-term relationships.
Research methodology built for decision-grade α-Ketoacid API insights through stakeholder validation, triangulated evidence, and value-chain mapping
The research methodology for this report is designed to produce decision-grade insights grounded in industry practice and verifiable evidence from market participants. It begins with a structured mapping of the α-ketoacid API value chain, including upstream raw material considerations, key synthesis and purification approaches, quality and regulatory expectations, and downstream demand drivers across pharmaceutical and nutrition-adjacent applications.Primary research is conducted through targeted engagement with knowledgeable stakeholders, such as manufacturers, distributors, procurement leaders, quality and regulatory professionals, and technical experts involved in process development and formulation. These conversations are used to validate observed trends, clarify buying criteria, and identify the operational constraints that shape supplier selection, qualification timelines, and switching costs.
Secondary research complements these insights through a rigorous review of public and proprietary materials permissible for use, including company disclosures, regulatory and standards frameworks, trade and customs information, scientific literature relevant to α-ketoacid chemistry and quality control, and documentation practices commonly required in regulated supply chains. Information is triangulated across sources to reduce bias and ensure consistency.
Finally, an internal validation process is applied to reconcile segmentation logic, regional dynamics, and competitive narratives. This includes consistency checks across interviews, documentation review, and value-chain analysis, with careful attention to avoiding unsupported assumptions. The result is a cohesive set of insights intended to support sourcing, partnership, and operational decisions without relying on speculative sizing claims.
Conclusion tying α-Ketoacid API success to resilient supply, rigorous quality execution, and segment-specific strategies amid policy and logistics change
The α-ketoacid API landscape is moving toward a more disciplined, compliance-forward model in which reliable execution and transparent quality systems carry as much weight as chemical capability. As buyers navigate evolving regulatory expectations, tighter documentation norms, and heightened supply risk, they are increasingly selecting partners that can demonstrate resilience, responsiveness, and control.At the same time, shifts in manufacturing strategy-ranging from process optimization to sustainability-driven improvements-are raising the bar for consistent impurity management and reproducibility. Trade and tariff uncertainty adds another layer, reinforcing the need for alternate sourcing strategies, validated routes, and contracting approaches that reduce volatility.
In this environment, the clearest path to durable advantage lies in aligning product and grade capabilities to the right applications, building regionally aware supply strategies, and investing in the operational maturity that accelerates customer qualification. Organizations that treat quality, documentation, and continuity as core commercial differentiators will be best positioned to earn long-term partnerships and withstand external disruption.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China a-Ketoacid APIs Market
Companies Mentioned
The key companies profiled in this α-Ketoacid APIs market report include:- Ajinomoto Co., Inc.
- Bachem Holding AG
- Cambrex Corporation
- Evonik Industries AG
- Hebei Yipin Pharmaceutical Co., Ltd.
- Lonza Group AG
- Merck KGaA
- Nanjing Lifenergy R & D Co., Ltd.
- Shandong Sinder Biotechnology Co., Ltd.
- Thermo Fisher Scientific Inc.
- Wuhan Yuancheng Gongchuang Technology Co., Ltd.
- Zhejiang NHU Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
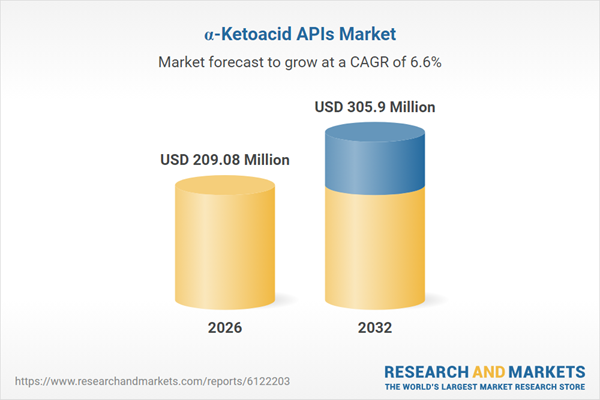
| Estimated Market Value ( USD | $ 209.08 Million |
| Forecasted Market Value ( USD | $ 305.9 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |